Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The synergistic impact of nanomaterials is critical for novel intracellular and/or subcellular drug delivery systems of minimal toxicity. This synergism results in a fundamental bio/nano interface interaction, which is discussed in terms of nanoparticle translocation, outer wrapping, embedding, and interior cellular attachment. The morphology, size, surface area, ligand chemistry and charge of nanoparticles all play a role in translocation.
- synergistic effect
- nanoparticles
- nanoalloy
- bimetallic nanoparticles
- green synthesis
1. Introduction
The immense beneficial impacts of science and technology on society and the general wellbeing of humankind are widely recognized. Within the field of nanotechnology, materials in a wide range of sizes and morphologies at the nanoscale have been produced. Nanoparticles (NPs) present unique properties that differ from those of their bulk counterparts due to quantum-size effects at nanometer size dimensions [1]. NPs in a variety of shapes and dimensionalities, ranging from one to three dimensions, have been extensively studied to investigate how quantum size effects impact their physical, chemical, and photothermal properties [2]. The morphology, size, and surface of metallic NPs can be uniquely tailored to specific medicinal and biological applications [3]. Their physicochemical properties change with the NP size, degree of NP concentration, etc., which may also affect their surface plasmonic characteristics [4]. Functionalized NPs have an intricate structural architecture, with a composition that can be divided up into three overlying levels: (a) the innermost core, the most fundamental part of the NP structure; (b) the shell that surrounds the core and may be adorned with ions, surfactants, polymers, and small molecules; and, finally, (c) the surface, functionalized with various ligands and peptides. This functionalization of nanoparticles can lead to a multitude of applications. Scientists from a variety of research areas have thus been drawn to NPs because of their rich, unique characteristics.
Nanosystems acting synergistically with cellular components have the potential to integrate diagnostic and therapeutic modalities, like cancer selectivity and bioimaging combined with targeted multimodal cancer therapies [5][6]. Nucleic acid conjugated nanomaterials (NACs) have proven to be a useful tool in cancer research [7][8][9][10][11]. They have the benefit of working as endogenous biomarkers with patient genetic information [12][13]. Graphene oxide/gold hybrid nanostructures (Au@GO NP-NACs) have emerged as an excellent platform for cancer diagnostics, as depicted in (Figure 1). Here, Au@GO NP-NAC nanosystems have been employed for site-specific gene/drug delivery, multimodal treatment, and noninvasive imaging, as illustrated in Figure 1a. Au@GO NP-NACs with unique surface-enhanced Raman spectroscopy (SERS) characteristics were created, and are shown in Figure 1b, for the noninvasive detection of oncogenes. This hybrid method uses Raman dye (Cy5)-labeled nucleic acid for a synergistic decrease in cancer treatment resistance. As shown in Figure 1, synergistic multimodal cancer diagnostics and gene suppression are potential mechanisms for cancer apoptosis (c). Silica-coated Au nanorods showed promise for cancer applications in recent research [14].
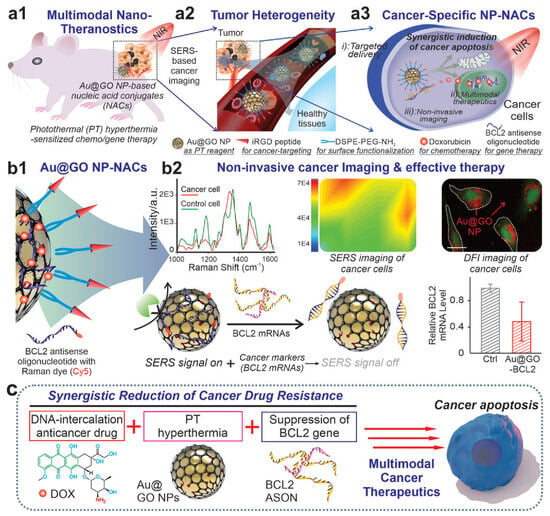
Figure 1. (a) The use of Au@GO NP-NACs showed photothermal hyperthermia-enhanced chemo/gene therapy for multimodal therapies. (b) For drug resistance, SERS imaging and gene therapy are employed. (c) The mechanism postulated for cancer apoptosis in synergistic multimodal cancer theragnostics. With permission, this figure was derived from reference [15].
The promise of nanotechnology goes beyond the applications in medicine and encompasses all aspects of human life. Here, people would like to mention parenthetically that the United Nations (UN) 2030 Agenda for Sustainable Development Goals (SDGs) was structured around four primary areas: social, economic, environmental, and governance areas [16][17]. The social pillar seeks to address issues such as poverty, poor nutrition, insufficient healthcare, the lack of access to safe water and sanitation, and insufficient sources of renewable energy. The economic pillar deals with the issues of sustainable urban development, innovation, infrastructure, and responsible manufacturing and consumption. The environmental pillar deals with our planet’s climate and marine and land life. Lastly, the governance pillar concerns itself with fostering peace, strong institutions, and partnerships. The UN has mandated the need to pool resources from many sources to achieve these SDGs [18]. In 2019, the UN Department of Economic and Social Affairs released a report titled, “The Future is Now—Science for Achieving Sustainable Development” [19]. Science, technology, and innovation are highlighted as key instruments for societal, healthcare, and economic advancement, and it is emphasized that new technology deployment should be prioritized [19].
Several studies in recent years have demonstrated the potential of nanomaterials to improve human health, water purification, and resource conservation. There have been numerous applications of metal-based nanostructures in the fields of electronics, gene therapy, drug delivery, and environmental remediation. These nanostructures offer exceptional properties, such as a higher surface area, surface energy, and chemical reactivity compared to macroscopic materials [20].
Furthermore, the improved optical and catalytic characteristics, among others, of bimetallic nanoparticles (BMNPs) relative to monometallic nanostructures have recently been investigated [21][22]. It has been determined that BMNPs can contribute to the achievement of several of the UN SDGs, such as excellent health and wellbeing, clean water and sanitation, and responsible consumption and production, with a focus on problems and solutions [23][24][25]. While there is research that suggests that metallic nanoparticles could be useful in Sustainability Agenda initiatives, no reviews exist that establish a direct correlation between these nanostructures and the UN SDGs [26][27][28][29].
2. Properties of Metallic Nanoparticles
Metallic nanostructures exhibit improved features, one of which is surface plasmonic behavior [30]. For instance, the size and structure of AgNPs determine how they interact with light [30]. The localized surface plasmon resonance (LSPR) is created when the conduction electrons surrounding the NP fluctuate in an orderly fashion due to the incident wavelength of light [31]. The development of nanostructured systems for detecting chemical and biological compounds was facilitated using this phenomenon for the electromagnetic augmentation of spectroscopic signals, such as surface-enhanced Raman scattering (SERS) [30]. The biomedical field has potential use for Ag and Au NPs beyond detection, particularly in antibacterial activities related to anticancer treatments [32]. The findings from the study by Soliman et al. [32] demonstrated that silver and gold nanoparticles have potential as antioxidant, anticancer, antibacterial, and antimicrobial agents.
An improved performance in the degradation of environmental toxins can be achieved using metallic nanostructures, which are famous for their catalytic activity [33]. Since copper (Cu) is both abundant and inexpensive, Cu NPs have shown promise as a material for degrading water contaminants such as methylene blue (MB) and Congo red (CR) [34]. In comparison to NaBH4, a commonly used reducing agent, Cu NPs have demonstrated superior catalytic characteristics in the reduction mechanism of the pollutants, resulting in the rapid and thorough elimination of CR and MB [34]. Along with their degrading capabilities, Cu NPs can be used electrochemically, allowing for the rapid and thorough elimination of these pollutants [34]. In addition, Cu NPs have been used electrochemically to diminish CO2-saturated aqueous solutions at room temperature and pressure [35].
2.1. Comparison between Mono- and Bimetallic Nanoparticles
Materials that combine two distinct metals using bimetallization techniques have improved and altered properties compared to those of a monometallic system. When contrasted with monometallic alternatives, BMNPs exhibit notable benefits [28]. The first benefit of bimetallization is an improvement in the system’s catalytic characteristics over those of monometallic nanoparticles (MNPs) [22]. For example, Pd-Ni, Pt-Ag, and Pd-Au nanowires (NWs) are bimetallic systems that enable electronic transitions within the NWs. These NWs have shown excellent electrochemical performance due to their greater surface area and acceptable stability [26][36]. Furthermore, when combinations of electrical, mechanical, functional, and structural modifications are introduced, they can cause synergistic effects when two metals are present [22].
BMNPs are more functional and have a wider range of applications than MNPs due to the regulated optical, electrical, plasmonic, thermal, and magnetic properties triggered by these interactions [37][38]. The thermal characteristics of a conductive ink containing BMNPs were studied by Yang et al. [34], who used differential scanning calorimetry (DSC) to determine the decomposition temperature of the ink. Adding Cu/Ag NPs to ink raises its decomposition temperature by around 30 °C compared to using just Ag NPs. In addition, it was demonstrated that the Cu:Ag weight ratio may be adjusted in a variable way to manage thermal stability. Anjo and colleagues [39] used a comparable strategy, testing the plasmonic and magnetic characteristics of Fe/Ag NPs produced via laser ablation. Varying the Fe:Ag composition ratio produced UV-Vis spectra that differed significantly, similar to Yang’s study [34]. Due to changes in particle shape and the overall composition of the system, the maximum absorbance wavelength was gradually shifted to the red end of the spectrum as the Fe:Ag ratio increased. All generated samples exhibited superparamagnetic behavior when tested for magnetic properties. However, the system with the optimal magnetic properties was the one that corresponded to a composition ratio of Fe50:Ag50 [39]. Additionally, Malik and colleagues reported on an Fe-Ag nanosystem, where the combination of Fe NPs’ reduction capacity and Ag NPs’ catalytic activity could result in the catalytic reduction of pollutants such as nitroaromatic chemicals [40]. Thus, systems based on noble metals can be systematically improved and used to create inexpensive solutions with improved thermal, plasmonic, magnetic, and catalytic capabilities by including transition metals, such as Cu and Fe. Finally, they are chemically stable and functionalize with ease [41]. Nanobranched bimetallic structures, such as AuCu, can improve the lower limit of detection (LOD) of biomarker detectors, such as glucose ones, with superior selectivity and stability [42]. It is believed that BMNPs could represent a more efficient way to achieve sustainability agenda goals because of these improved attributes.
2.2. Structure and Characteristics of Bimetallic Nanoparticles
The classification of bimetallic systems is heavily influenced by their structure, architecture, and composition [43]. Noble metal BMNPs have high catalytic characteristics and strong plasmon resonances because of their electronic configuration, which is determined by their composition [44]. Iron (Fe), nickel (Ni), gold (Au), cobalt (Co), and silver (Ag) are the most popular metals utilized in bimetallic systems.
The composition of noble metal BMNPs determines their electronic configuration, which, in turn, determines their high catalytic properties and allows the display of strong plasmon resonances [44]. As mentioned above, among the most popular metals utilized in bimetallic systems are iron (Fe), nickel (Ni), gold (Au), and cobalt (Co) [44]. The outermost n-electrons and unsaturated (n − 1) d electron shell of these metals contribute to their catalytic and magnetic characteristics, respectively [45]. Finally, since noble metals are expensive due to their low abundance and dispersed distribution in the Earth’s crust [46], combining them with transition metals makes BMNPs more affordable.
3. Nanoalloy Synergistic Applications
3.1. Gold Nanoalloys
Noble metal nanoparticles have outstanding physicochemical characteristics and are suitable for biomedical applications [47][48]. Both silver (Ag) and gold (Au) nanoparticles have applications in biology, health, and biochemistry [49][50][51]. The antibacterial activity of Ag nanoparticles is well known and used in biomedical and consumer products [52][53][54][55][56][57]. The toxicity of Ag nanoparticles, however, is a major concern in the biomedical field [58][59]. For this reason, Au nanoparticles are preferable due to their biocompatible nature [60] and have been applied to drug delivery, biological imaging, and cancer therapy [61][62][63][64][65][66][67][68][69]. Hybrid (Cu and Au) tripod nanocrystals have been examined both empirically and theoretically by our research group; upon NIR laser irradiation, researchers discovered a distinct photothermal-effect-based anticancer treatment [6].
In principle, a hybrid Au and Ag nanoalloy can enhance biomedical properties, such as Ag NP toxicity toward bacteria or cells and Au NP biocompatibility properties [70]. A variety of techniques to manufacture Au–Ag nanoalloys have been studied. UV irradiation, sol–gel techniques, and wet chemical synthesis are examples of synthetic procedures [71][72][73][74][75][76]. So far, laser irradiation has been employed for the synthesis of Au–Ag nanoalloys [77]. It has also been reported that the ultrasonication of individual Ag and Au nanoparticles [78] or refluxing with oleylamine [79][80] may produce Au shell/Ag core nanoalloys.
According to Georgios A. Sotiriou et al. [81], surface oxidation and the leaching of Ag ions can be avoided through the presence of Au in the production of Ag nanoparticles. Compared to pure Au nanoparticles, inexpensive and biocompatible Au–Ag nanoalloys have outstanding plasmonic characteristics. Nanoalloys are becoming attractive options in medical sectors because of their strong plasmonic characteristics. Figure 2a depicts the release profile of Ag ions from Ag–Au nanoalloys. The 17 mg L1 Ag ions produced by pure Ag nanoparticles account for around 17% of the overall mass. The discharge of Ag ions increases as the Au concentration decreases. The differential plasmon peak observed in water and ethanol is shown in Figure 2b. The plasmon peak position difference increases as the Au concentration decreases, making it safer (Figure 2c).
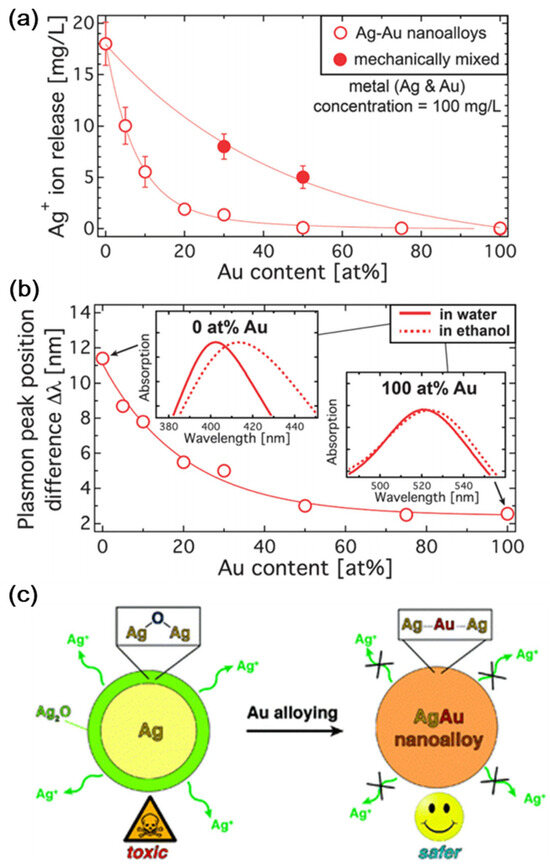
Figure 2. (a) The release profile of Ag ions in aqueous solution. (b) Plasmon peak of nanoalloys observed in ethanol and water medium. (c) Au addition reduces surface oxidation and hazardous Ag+ ion leaching. With permission, this figure was taken from reference [81].
Because of its outstanding optical characteristics and high photothermal transition efficiency, the plasmon resonance of metallic nanoparticles plays an important role in disease diagnostics, biological sensing [82], and other medicinal sectors [3]. Various research studies have focused on Ag and Au in nanoalloys [83][84]. However, under typical experimental conditions, the surface of a Ag-nanoparticle can be reduced [85], reducing its plasmonic activity [86]. Surface oxidation reduced by Au-Ag nanoalloys is useful for SERS applications [87][88][89][90]. As a result, the Au-Ag nanoalloy system is a promising platform to produce non-toxic and low-cost plasmonic nanomaterials.
3.2. Magnetic Nanoalloys
Magnetic nanoparticles provide an excellent opportunity for hyperthermia ablation and magnetic tumor separation, and act as contrast enhancers in magnetic resonance imaging (MRI) [91][92][93][94][95][96][97][98][99][100][101]. This is a noninvasive, powerful biological imaging technique, that relies on protons in lipids and water molecules for its signal. Magnetic nanoparticles can cause a large contrast between diseased and normal organ tissue in biological imaging, acting as MRI image enhancing agents [102][103]. Magnetic nanoparticles (FeCo, MnFe2O4, Fe2O3, Fe3O4) are common enhancing agents used in MRI imaging of the liver, spleen, gastrointestinal system, and bone marrow [104][105]. Apart from its widespread use, magnetic iron oxide has several drawbacks, including rapid clearance by phagocytic cells and the prevention of tissue penetration and trans-endothelial transit [106]. When targeting an organ, it is critical to produce an MRI contrast agent with increased selectivity, tissue delineation, and longer intravascular retention.
Magnetic nanoalloys have been shown to have longer blood circulation times, improving the possibility of interaction with specific tissues [107]. The biocompatible and inert shell afforded by silica is considered a popular coating material for magnetic nanoalloys. The silica coating may preserve chemicals from deterioration and can facilitate the adorning of the NP surface with functional chemical groups [108][109][110]. Surfaces containing chemical groups provide several advantages, such as solubility and stability in an aqueous medium for biomedical applications [111]. This improves the binding ability of biological compounds and the effectiveness of cell internalization for the targeted administration of medicines and MRI applications [112][113].
Platinum–iron (Pt-Fe) nanoalloys offer a unique form of magnetic nanomaterial because of their high magneto-crystalline anisotropy and Curie temperature [114]. Various synthesis techniques have been investigated in the past to generate platinum iron nanoalloys with different sizes, shapes, and stoichiometries. The co-reduction of platinum and iron materials via low-pressure emulsion protocols [41][115][116][117][118], the high-pressure polyol route, and the photothermal technique are used for the synthesis of magnetic nanoalloys [38][119][120][121][122][123][124].
Medical applications for magnetic nanoalloys have been studied, including T2 MRI contrast agents [125][126] and hyperthermia ablation [127][128], as well as magnetic separation [129][130]. However, NP toxicity remains a serious issue that prevents their widespread use in biological treatment and diagnostics [131][132]. Due to the retention effect and increased permeability, Fe3O4@Au NPs resulted in high tumor accumulation. As shown in Figure 3, Fe3O4@Au NPs were utilized for NIR laser-induced photothermal synergistic treatment and multimodal imaging systems [126].
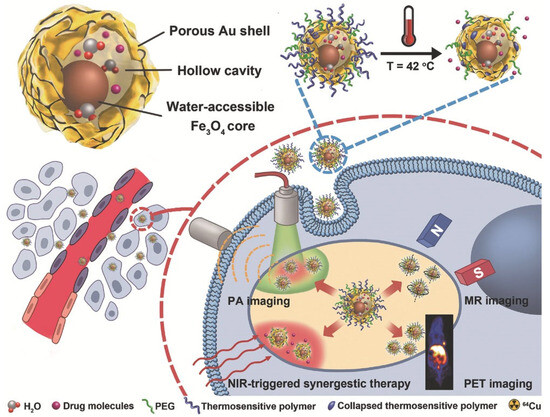
Figure 3. Fe3O4@Au NPs for NIR-triggered photothermal synergistic treatment and MR/PA/PET multimodal imaging are shown. With permission, this figure was derived from ref. [126].
4. Mechanism of Nanoparticle–Cell Interaction
In 1857, Michael Faraday studied the preparation and properties of colloidal suspensions of “Ruby” gold, ref. [133] considered to be the first experiment on nanoparticles in modern times. Today, there are a wide range of applications for nanoparticles [134][135][136][137][138][139][140][141][142][143]. Metallic nanoparticles, in contrast to their bulk counterparts, show defined activities that are useful in a variety of biomedical applications. High dangling bonds, electron storage capacity [144], the presence of edges and corners, a high surface energy, surface plasmon resonances (SPR), and a high surface area-to-volume ratio are among the functions [145][146]. Different processes, such as biological, physical, and chemical techniques, can create metallic nanoparticles with diverse shapes and sizes [147][148][149].
Nanoscience has produced different nanomaterials with distinct ligands and groups to display dissimilar functionalities and characteristics. Nanomaterials, because of their tiny size, might effectively react with the biological cell membrane and intracellular fluid [150]. Nanoparticles are most used in antimicrobial processes, catalysis, cancer treatment, imaging, diagnostics, and medication delivery [151][152][153]. Nanoparticles may be partially embedded in cell membranes, exposing them to both extracellular fluid and cytosol. This structure mimics that of a transmembrane protein. Using nanoparticles containing alkyl groups, researchers showed the existence of embedment structures [154]. However, if large numbers of nanoparticles are delivered inside a cell, they may damage subcellular organelles and the cellular membrane itself, thus causing cytotoxicity.
Embedment, inner attachment, free translocation, and the outside wrap are four different ways for the cell membrane and nanoparticles to interact (Figure 4). In terms of the outer wrap, nanoparticles only adhere to the cell membrane’s exterior surface and cannot permeate even a small portion of the membrane. When nanoparticles are large and adorned with a low charge, this structure can be seen (Figure 4). The plasma membrane of the cells contains microdomains that are enriched in certain cholesterol, gangliosides, and glycosphingolipids that form membrane/lipid rafts. Membrane/lipid rafts have myriad functions, including the regulation of cellular polarity and the organization of sorting and trafficking mechanisms. These rafts are also important for forming platforms for intracellular cytoskeletal binding and extracellular matrix adhesion to the plasma membrane. Furthermore, they are involved in the generation of signaling events and constitute the sites where nanoparticles enter the cells.
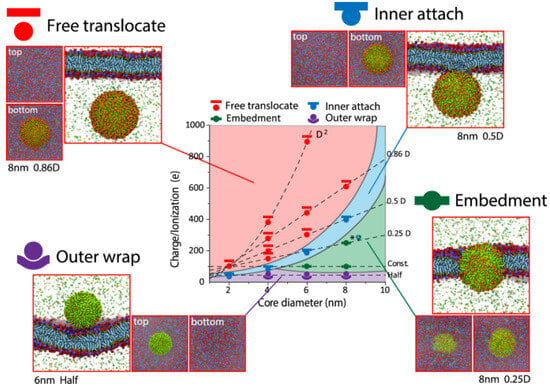
Figure 4. Hydrophobic ligands adorn nanoparticles with various surface ionization/charge and core sizes (alkyls). With permission, this figure was taken from ref. [154].
Nanoparticle interactions with the cellular membrane are achieved through key physicochemical properties such as size, surface charge, and ligand chemistry [154]. Nanoparticles with a diameter less than 1 nm, such as ions or water molecules, enter the cellular membrane via permeation. Larger-size nanoparticles enter the cell membrane through endocytosis, which includes receptor-mediated endocytosis, pinocytosis, and phagocytosis. Medium-size nanoparticles lie on the boundary between endocytosis and permeation. Proteins (3–20 nm) go through complex interaction with cell membranes. Lin et al. [154] found nanoparticles with inner attachment to cell membranes, which results in nanoparticles being exposed to the intracellular fluid as they are attached to the inner surface of the cell membrane via focal adhesion [154]. Only when nanoparticles are coated with hydrophobic groups and for certain size–charge combinations can this structure be seen [154]. Their suspension is then available within the intracellular fluid for free translocation. This situation can arise when nanoparticles are tiny and adorned with a high charge [154].
5. Green Synthesis Trends in Bimetallic Nanoparticles
Combining the principles of green chemistry with those of traditional physicochemical methods, we can develop biologically based synthesis processes that are both safe and environmentally friendly and incorporate nanomaterials into industrial processes on a large scale, while reducing the likelihood of harm to humans and the environment [155]. The synthesis of BMNPs in an eco-friendly way must adhere to green chemistry techniques: using solvents and reducing agents that are not harmful to the environment and capping agents that are not poisonous [156]. The field of green nanotechnology came into being when these concepts of green chemistry were applied to the production of nanomaterials [157]. Therefore, BMNPs synthesized and applied using green nanotechnology in sustainability science have demonstrated efficacy against water and soil contamination and antibacterial activity against pathogen bacterial strains in the food and health industries.
Biomolecules derived from many sources, including plants, algae, bacteria, fungi, and organic waste products, such as fruit peels, are commonly utilized as agents for reduction and stabilization [158]. Metallic salts can be reduced in either an intracellular or extracellular environment in bacterial- and fungal-mediated production. Biomedical and technical applications can benefit from zero-valence metallic structures that can be produced through the bacteria-mediated green synthesis of BMNPs. Fungus-mediated syntheses are preferable over bacterium-mediated ones because they can be used in speedier, scaled-up processes [159][160]. Metal NP syntheses mediated by plants are among the most popular methods. It is common practice to begin these procedures by obtaining a plant extract in liquid form; this yields a fine powder that needs to be washed and dried before synthesis [161]. Because plant leaves include various functional groups and phyto-chemicals that work as reducing and stabilizing agents, these interactions are beneficial [162]. For the biomolecule-mediated production of metallic NPs, typical ingredients include poly-ethylene glycol [163] and starch [164]. Finally, one of the less investigated forms of synthesis is that which is mediated by waste materials.
6. Conclusions
In shaping the translocation outcome, the physiochemical properties of the nanoparticles along with their synergistic effects play a vital role. Synergistically, they trigger initial pore nucleation for NP translocation. The impact of nanoparticles acting synergistically promise to advance Green Chemistry and Sustainability, as required by the UN sustainability agenda. The study herein reviewed the literature on the synergistic impact of nanoparticles on green nanotechnology. The synergistic effect between the nanocomposite, nanoalloy, and nanoparticles can be conceptualized theoretically. The interactions between various nanoalloys, nanocomposites, and nanoparticles have been discussed. However, the details of the underlying processes need further investigation, as the field of green nanotechnology is still in its infancy. The focus of the scientific community on the use of nanotechnology for technical and consumer applications and in medical diagnostics and treatment during the last decades has resulted in the establishment of a substantial database. We need to further deepen our knowledge on the interaction of various nanoparticles with specific biological barriers and compartments in optimizing internalization and site-specific drug release.
References
- Amarnath, C.A.; Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K.; Paik, U. Nanohybridization of low-dimensional nanomaterials: Synthesis, classification, and application. Crit. Rev. Solid State Mater. Sci. 2013, 38, 1–56.
- Xu, L.; Ma, W.; Wang, L.; Xu, C.; Kuang, H.; Kotov, N.A. Nanoparticle assemblies: Dimensional transformation of nanomaterials and scalability. Chem. Soc. Rev. 2013, 42, 3114–3126.
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779.
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262.
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672.
- Nanda, S.S.; Hembram, K.P.S.S.; Lee, J.K.; Kim, K.; Selvan, S.T.; Yi, D.K. Experimental and theoretical structural characterization of Cu–Au tripods for photothermal anticancer therapy. ACS Appl. Nano Mater. 2019, 2, 3735–3742.
- Kundu, A.; Nandi, S.; Nandi, A.K. Nucleic acid based polymer and nanoparticle conjugates: Synthesis, properties and applications. Prog. Mater. Sci. 2017, 88, 136–185.
- Thi, E.P.; Mire, C.E.; Lee, A.C.H.; Geisbert, J.B.; Zhou, J.Z.; Agans, K.N.; Snead, N.M.; Deer, D.J.; Barnard, T.R.; Fenton, K.A.; et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015, 521, 362.
- Cutler, J.I.; Auyeung, E.; Mirkin, C.A. Spherical nucleic acids. J. Am. Chem. Soc. 2012, 134, 1376–1391.
- Stengel, D.; Jörgensen, A.M.; Polidori, I.; Kapitza, P.; Ricci, F.; Bernkop-Schnürch, A. The power of sulfhydryl groups: Thiolated lipid-based nanoparticles enhance cellular uptake of nucleic acids. J. Colloid Interface Sci. 2024, 654, 1136–1145.
- Haizan, I.; Park, D.H.; Choi, M.Y.; Lee, H.; Choi, J.H. Nanomaterials-Based Exosomes for the Diagnostics and Drug Deliveries of Central Nervous System Diseases. BioChip J. 2023, 17, 293–307.
- Lauschke, V.M.; Zhou, Y.; Ingelman-Sundberg, M. Pharmacogenomics beyond single common genetic variants: The way forward. Annu. Rev. Pharmacol. Toxicol. 2023, 64, 33–51.
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat. Rev. Drug Discov. 2023, 22, 185–212.
- Wang, T.; Yeom, K.S.; Nanda, S.S.; An, S.S.A.; Yi, D.K. Cancer cell growth in the near infrared region by using silica coated gold nanorods. Nano 2020, 15, 2050001.
- Yang, L.; Kim, T.H.; Cho, H.Y.; Luo, J.; Lee, J.M.; Chueng, S.T.D.; Hou, Y.; Yin, P.T.T.; Han, J.; Kim, J.H.; et al. Hybrid graphene-gold nanoparticle-based nucleic acid conjugates for cancer-specific multimodal imaging and combined therapeutics. Adv. Funct. Mater. 2021, 31, 2006918.
- Galan-Ladero, M.M.; Sarmento, M.; Marques, S. Social Marketing to achieve the Sustainable Development Goals (SDGs) in 2030 Agenda by the United Nations. Int. Rev. Public Nonprofit Mark. 2023, 20, 521–527.
- Mangukiya, R.D.; Sklarew, D.M. Analyzing three pillars of sustainable development goals at sub-national scales within the USA. World Dev. Sustain. 2023, 2, 100058.
- Walsh, P.P.; Murphy, E.; Horan, D. The role of science, technology and innovation in the UN 2030 agenda. Technol. Forecast. Soc. Change 2020, 154, 119957.
- United Nations. The future is now—Science for achieving sustainable development. In Global Sustainable Development Report by the Independent Group of Scientists; United Nations: New York, NY, USA, 2019.
- Bairagi, S.; Kamali, M.R. Review on green biomass-synthesized metallic nanoparticles and composites and their photocatalytic water purification applications: Progress and perspectives. Chem. Eng. J. Adv. 2023, 14, 100460.
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Nieto-Argüello, A.; Lomelí-Marroquín, D.; Vélez-Escamilla, L.Y.; Cholula-Díaz, J.L.; García-Martín, J.M.; Webster, T. Bimetallic nanoparticles for biomedical applications: A review. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Springer: Cham, Switzerland, 2020; pp. 397–434.
- Blosi, M.; Ortelli, S.; Costa, A.L.; Dondi, M.; Lolli, A.; Andreoli, S.; Benito, P.; Albonetti, S. Bimetallic nanoparticles as efficient catalysts: Facile and green microwave synthesis. Materials 2016, 9, 550.
- Ren, G.; Wan, K.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.; Wei, G. Recent advance in biomass membranes: Fabrication, functional regulation, and antimicrobial applications. Carbohydr. Polym. 2023, 305, 120537.
- Kumar, N.; Gusain, R.; Pandey, S.; Ray, S.S. Hydrogel Nanocomposite Adsorbents and Photocatalysts for Sustainable Water Purification. Adv. Mater. Interfaces 2023, 10, 2201375.
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The barrier properties of sustainable multiphase and multicomponent packaging materials: A review. Prog. Mater. Sci. 2023, 133, 101071.
- Larrañaga-Tapia, M.; Betancourt-Tovar, B.; Videa, M.; Antunes-Ricardo, M.; Cholula-Díaz, J.L. Green synthesis trends and potential applications of bimetallic nanoparticles towards the sustainable development goals 2030. Nanoscale Adv. 2024, 6, 51–71.
- Shrivastava, P.; Jain, V.K.; Nagpal, S. Nanoparticle intervention for heavy metal detection: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100667.
- Idris, D.S.; Roy, A. Synthesis of Bimetallic Nanoparticles and Applications—An Updated Review. Crystals 2023, 13, 637.
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Trimetallic nanoparticles: Greener synthesis and their applications. Nanomaterials 2020, 10, 1784.
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780.
- Cholula-Díaz, J.L.; Lomelí-Marroquín, D.; Pramanick, B.; Nieto-Argüello, A.; Cantú-Castillo, L.A.; Hwang, H. Synthesis of colloidal silver nanoparticle clusters and their application in ascorbic acid detection by SERS. Colloids Surf. B Biointerfaces 2018, 163, 329–335.
- Geleta, G.S. A colorimetric aptasensor based on two dimensional (2D) nanomaterial and gold nanoparticles for detection of toxic heavy metal ions: A review. Food Chem. Adv. 2023, 2, 100184.
- Soliman, M.K.; Salem, S.S.; Abu-Elghait, M.; Azab, M.S. Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl. Biochem. Biotechnol. 2023, 195, 1158–1183.
- Yang, W.; Wang, C.; Arrighi, V. One step preparation of copper–silver self-catalyzed hybrid conductive ink with reduced sintering temperature for flexible electronics. J. Mater. Sci. Mater. Electron. 2019, 30, 11607–11618.
- Alahdal, F.A.; Qashqoosh, M.T.; Manea, Y.K.; Mohammed, R.K.; Naqvi, S. Green synthesis and characterization of copper nanoparticles using Phragmanthera austroarabica extract and their biological/environmental applications. Sustain. Mater. Technol. 2023, 35, e00540.
- Gomez, F.J.; Chumanov, G.; Silva, M.F.; Garcia, C.D. CO2 reduction using paper-derived carbon electrodes modified with copper nanoparticles. RSC Adv. 2019, 9, 33657–33663.
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175.
- Behera, A.; Mittu, B.; Padhi, S.; Patra, N.; Singh, J. Bimetallic nanoparticles: Green synthesis, applications, and future perspectives. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 639–682.
- Anjo, L.; Khajehnezhad, A.; Sari, A.H.; Sebt, S.A.; Ismail, M.M. Coexistence of Plasmonic and Magnetic Properties in Bimetallic Fe/Ag Nanoparticles Synthesized by Pulsed Laser Ablation. Plasmonics 2022, 17, 941–948.
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile one-pot green synthesis of Ag–Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021, 12, 455–470.
- Rajeev, R.; Datta, R.; Varghese, A.; Sudhakar, Y.N.; George, L. Recent advances in bimetallic based nanostructures: Synthesis and electrochemical sensing applications. Microchem. J. 2021, 163, 105910.
- Wang, R.; Tang, T.; Lu, G.; Zheng, Z.; Huang, K.; Li, H.; Tao, X.; Yin, H.; Shi, Z.; Lin, Z.; et al. Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems. Sci. Total Environ. 2019, 661, 18–26.
- Ngamaroonchote, A.; Sanguansap, Y.; Wutikhun, T.; Karn-Orachai, K. Highly branched gold–copper nanostructures for non-enzymatic specific detection of glucose and hydrogen peroxide. Microchim. Acta 2020, 187, 559.
- Srinoi, P.; Chen, Y.T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106.
- Das, P.; Borthakur, P.; Boruah, P.K.; Das, M.R. Peroxidase mimic activity of Au-Ag/l-Cys-rGO nanozyme toward detection of Cr (VI) ion in water: Role of 3, 3′, 5, 5′-tetramethylbenzidine adsorption. J. Chem. Eng. Data 2019, 64, 4977–4990.
- Jiang, L.Y.; Na, L. (Eds.) Membrane-Based Separations in Metallurgy: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017.
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold nanoparticles: Past, present, and future. Langmuir 2009, 25, 13840–13851.
- Kaushal, S.; Nanda, S.S.; Samal, S.; Yi, D.K. Strategies for the development of metallic-nanoparticle-based label-free biosensors and their biomedical applications. ChemBioChem 2020, 21, 576–600.
- Kaushal, S.; Nanda, S.S.; Yi, D.K.; Ju, H. Effects of aspect ratio heterogeneity of an assembly of gold nanorod on localized surface plasmon resonance. J. Phys. Chem. Lett. 2020, 11, 5972–5979.
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248.
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908.
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668.
- Liu, H.L.; Dai, S.A.; Fu, K.Y.; Hsu, S.H. Antibacterial properties of silver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethane. Int. J. Nanomed. 2010, 5, 1017–1028.
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175.
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653.
- Mohamed, R.M.; Fawzy, E.M.; Shehab, R.A.; Abdel-Salam, M.O.; Salah El Din, R.A.; Abd El Fatah, H.M. Production, characterization, and cytotoxicity effects of silver nanoparticles from Brown alga (Cystoseira myrica). J. Nanotechnol. 2022, 2022, 6469090.
- Karakaş, İ. Some biological potential of silver nanoparticles synthesized from Ocimum basilicum L. GSC Biol. Pharm. Sci. 2023, 22, 107–113.
- Rajan, R.; Huo, P.; Chandran, K.; Dakshinamoorthi, B.M.; Yun, S.I.; Liu, B. A review on the toxicity of silver nanoparticles against different biosystems. Chemosphere 2022, 292, 133397.
- Choudhary, A.; Singh, S.; Ravichandiran, V. Toxicity, preparation methods and applications of silver nanoparticles: An update. Toxicol. Mech. Methods 2022, 32, 650–661.
- Taha, R.H. Green synthesis of silver and gold nanoparticles and their potential applications as therapeutics in cancer therapy; A review. Inorg. Chem. Commun. 2022, 143, 109610.
- Milan, J.; Niemczyk, K.; Kus-Liśkiewicz, M. Treasure on the Earth—Gold nanoparticles and their biomedical applications. Materials 2022, 15, 3355.
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold nanoparticles: Synthesis methods, functionalization and biological applications. J. Clust. Sci. 2023, 34, 705–725.
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2022, 3, 792–828.
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824.
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2021, 33, 1–16.
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787.
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480.
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685.
- Yu, Y.; Yang, T.; Sun, T. New insights into the synthesis, toxicity and applications of gold nanoparticles in CT imaging and treatment of cancer. Nanomedicine 2020, 15, 1127–1145.
- Tang, J.; Shi, H.; Ma, G.; Luo, L.; Tang, Z. Ultrasmall Au and Ag nanoclusters for biomedical applications: A review. Front. Bioeng. Biotechnol. 2020, 8, 1019.
- Babawale, O.E.; Gundlach, L. Fabrication of Large-Area Fully Alloyed Ag/Au Nanoparticle Arrays. ACS Appl. Nano Mater. 2023, 6, 21866–21875.
- Petkov, V.; Prasai, B.; Ren, Y.; Shan, S.; Luo, J.; Joseph, P.; Zhong, C.J. Solving the nanostructure problem: Exemplified on metallic alloy nanoparticles. Nanoscale 2014, 6, 10048–10061.
- Chen, D.H.; Chen, C.J. Formation and characterization of Au–Ag bimetallic nanoparticles in water-in-oil microemulsions. J. Mater. Chem. 2002, 12, 1557–1562.
- Devarajan, S.; Bera, P.; Sampath, S. Bimetallic nanoparticles: A single step synthesis, stabilization, and characterization of Au–Ag, Au–Pd, and Au–Pt in sol–gel derived silicates. J. Colloid Interface Sci. 2005, 290, 117–129.
- Gonzalez, C.M.; Liu, Y.; Scaiano, J.C. Photochemical strategies for the facile synthesis of gold−silver alloy and core−shell bimetallic nanoparticles. J. Phys. Chem. C 2009, 113, 11861–11867.
- Grade, S.; Eberhard, J.; Jakobi, J.; Winkel, A.; Stiesch, M.; Barcikowski, S. Alloying colloidal silver nanoparticles with gold disproportionally controls antibacterial and toxic effects. Gold Bull. 2014, 47, 83–93.
- Neumeister, A.; Jakobi, J.; Rehbock, C.; Moysig, J.; Barcikowski, S. Monophasic ligand-free alloy nanoparticle synthesis determinants during pulsed laser ablation of bulk alloy and consolidated microparticles in water. Phys. Chem. Chem. Phys. 2014, 16, 23671–23678.
- Taylor, U.; Tiedemann, D.; Rehbock, C.; Kues, W.A.; Barcikowski, S.; Rath, D. Influence of gold, silver and gold–silver alloy nanoparticles on germ cell function and embryo development. Beilstein J. Nanotechnol. 2015, 6, 651–664.
- Wang, C.; Peng, S.; Chan, R.; Sun, S. Synthesis of AuAg Alloy Nanoparticles from Core/Shell-Structured Ag/Au. Small 2009, 5, 567–570.
- Radziuk, D.V.; Zhang, W.; Shchukin, D.; Möhwald, H. Ultrasonic alloying of preformed gold and silver nanoparticles. Small 2010, 6, 545–553.
- Sotiriou, G.A.; Etterlin, G.D.; Spyrogianni, A.; Krumeich, F.; Leroux, J.C.; Pratsinis, S.E. Plasmonic biocompatible silver–gold alloyed nanoparticles. Chem. Commun. 2014, 50, 13559–13562.
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453.
- Cortie, M.B.; McDonagh, A.M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 2011, 111, 3713–3735.
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830.
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246.
- Li, Y.; Deng, Z. Ag ion soldering: An emerging tool for sub-nanomeric plasmon coupling and beyond. Acc. Chem. Res. 2019, 52, 3442–3454.
- Lee, H.; Yoo, Y.; Kang, T.; In, J.; Seo, M.K.; Kim, B. Topotaxial Fabrication of Vertical AuxAg1−x Nanowire Arrays: Plasmon-Active in the Blue Region and Corrosion Resistant. Small 2012, 8, 1527–1533.
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983.
- Sotiriou, G.A.; Meyer, A.; Knijnenburg, J.T.; Panke, S.; Pratsinis, S.E. Quantifying the origin of released Ag+ ions from nanosilver. Langmuir 2012, 28, 15929–15936.
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10.
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278.
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-el-salhi, A.; Elaissari, A. Magnetic nanoparticles: From synthesis to theranostic applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306.
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448.
- Ferreira, M.; Sousa, J.; Pais, A.; Vitorino, C. The role of magnetic nanoparticles in cancer nanotheranostics. Materials 2020, 13, 266.
- Baki, A.; Wiekhorst, F.; Bleul, R. Advances in magnetic nanoparticles engineering for biomedical applications—A Review. Bioengineering 2021, 8, 134.
- Díez, A.G.; Rincón-Iglesias, M.; Lanceros-Méndez, S.; Reguera, J.; Lizundia, E. Multicomponent magnetic nanoparticle engineering: The role of structure-property relationship in advanced applications. Mater. Today Chem. 2022, 26, 101220.
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic nanoparticles in biology and medicine: Past, present, and future trends. Pharmaceutics 2021, 13, 943.
- Farinha, P.; Coelho, J.M.; Reis, C.P.; Gaspar, M.M. A comprehensive updated review on magnetic nanoparticles in diagnostics. Nanomaterials 2021, 11, 3432.
- Brito, B.; Price, T.W.; Gallo, J.; Bañobre-López, M.; Stasiuk, G.J. Smart magnetic resonance imaging-based theranostics for cancer. Theranostics 2021, 11, 8706.
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003.
- Cheng, H.W.; Tsao, H.Y.; Chiang, C.S.; Chen, S.Y. Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 2021, 10, 2001451.
- Wallyn, J.; Anton, N.; Akram, S.; Vandamme, T.F. Biomedical imaging: Principles, technologies, clinical aspects, contrast agents, limitations and future trends in nanomedicines. Pharm. Res. 2019, 36, 78.
- Li, H.; Meade, T.J. Molecular magnetic resonance imaging with Gd (III)-based contrast agents: Challenges and key advances. J. Am. Chem. Soc. 2019, 141, 17025–17041.
- Yan, G.P.; Robinson, L.; Hogg, P. Magnetic resonance imaging contrast agents: Overview and perspectives. Radiography 2007, 13, e5–e19.
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390.
- Bulte, J.W.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499.
- McNamara, K.; Tofail, S.A. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995.
- Yi, D.K.; Lee, S.S.; Papaefthymiou, G.C.; Ying, J.Y. Nanoparticle architectures templated by SiO2/Fe2O3 nanocomposites. Chem. Mater. 2006, 18, 614–619.
- Yi, D.K.; Lee, S.S.; Ying, J.Y. Synthesis and applications of magnetic nanocomposite catalysts. Chem. Mater. 2006, 18, 2459–2461.
- Yi, D.K.; Selvan, S.T.; Lee, S.S.; Papaefthymiou, G.C.; Kundaliya, D.; Ying, J.Y. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J. Am. Chem. Soc. 2005, 127, 4990–4991.
- Duong, H.K.; Abdibastami, A.; Gloag, L.; Barrera, L.; Gooding, J.J.; Tilley, R.D. A guide to the design of magnetic particle imaging tracers for biomedical applications. Nanoscale 2022, 14, 13890–13914.
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnol. 2022, 20, 305.
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Toxicological aspects of iron oxide nanoparticles. In Nanotoxicology in Safety Assessment of Nanomaterials; Springer International Publishing: Cham, Switzerland, 2022; pp. 303–350.
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic nanoparticles: Synthesis, anisotropy, and applications. Chem. Rev. 2021, 123, 3904–3943.
- Capelli, S.; Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Iron as modifier of Pd and Pt-based catalysts for sustainable and green processes. Inorganica Chim. Acta 2022, 535, 120856.
- McNamara, K.; Tofail, S.A.; Thorat, N.D.; Bauer, J.; Mulvihill, J.J. Biomedical applications of nanoalloys. In Nanoalloys, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–432.
- Coviello, V.; Forrer, D.; Amendola, V. Recent developments in plasmonic alloy nanoparticles: Synthesis, modelling, properties and applications. ChemPhysChem 2022, 23, e202200136.
- Dik, G.; Ulu, A.; Ates, B. Medicinal and Biological Application of Magnetic Alloy Nanoparticles and Their Polymer Nanocomposites. In Handbook of Magnetic Hybrid Nanoalloys and their Nanocomposites; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–27.
- Semaltianos, N.G.; Karczewski, G. Laser synthesis of magnetic nanoparticles in liquids and application in the fabrication of polymer–nanoparticle composites. ACS Appl. Nano Mater. 2021, 4, 6407–6440.
- Thomas, S.; Nochehdehi, A.R. (Eds.) Handbook of Magnetic Hybrid Nanoalloys and Their Nanocomposites; Springer Nature: Berlin/Heidelberg, Germany, 2022.
- Simon, J.; Nampoori, V.P.N.; Kailasnath, M. Concentration dependent thermo-optical properties and nonlinear optical switching behavior of bimetallic Au-Ag nanoparticles synthesized by femtosecond laser ablation. Opt. Laser Technol. 2021, 140, 107022.
- Aslan, N.; Koç, M.M. X-ray Computed Tomography and Magnetic Resonance Imaging Applications of Magnetic Nanoalloys and Nanocomposites. In Handbook of Magnetic Hybrid Nanoalloys and their Nanocomposites; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–20.
- Gabbani, A.; Fantechi, E.; Albino, M.; Sangregorio, C.; Pineider, F. Intermetallic Au3LixM1−x (M = Fe, Ni or Co) nanoalloys: Effect of synthetic conditions on the composition and order-disorder transition. Inorganica Chim. Acta 2023, 554, 121545.
- Abduljawad, M. Synthesis and Functionalization of Hybrid Magnetic Nanoparticle Composites for Energy Conversion, Light Harvesting and Optical and Biomedical Applications. Doctoral Dissertation, The University of Arizona, Tucson, AZ, USA, 2021.
- Basagni, A.; Torresan, V.; Marzola, P.; van Raap, M.B.F.; Nodari, L.; Amendola, V. Structural evolution under physical and chemical stimuli of metastable Au–Fe nanoalloys obtained by laser ablation in liquid. Faraday Discuss. 2023, 242, 286–300.
- Lin, L.S.; Yang, X.; Zhou, Z.; Yang, Z.; Jacobson, O.; Liu, Y.; Yang, A.; Niu, G.; Song, J.; Yang, H.H.; et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 2019, 9, 7200.
- Singh, S.; Seehra, M.S. Testing the validity of the core-shell-surface layer model on the size dependence of effective magnetic anisotropy in magnetic nanoparticles. Front. Mater. 2022, 9, 1050600.
- Tran, H.B.; Matsushita, Y.I. Temperature and size dependence of energy barrier for magnetic flips in L10 FePt nanoparticles: A theoretical study. Scr. Mater. 2024, 242, 115947.
- Zhang, Z.; He, P.; Ma, W.; Zuo, P.; Liu, X.; Zhuang, Q. Freely Tailorable Yolk-Shell Encapsulation: Versatile Applications in Ultralow-k Dielectric, Drug Delivery Systems, and Catalysts. Adv. Funct. Mater. 2023, 33, 2302212.
- Liu, Y.; Wu, P.C.; Guo, S.; Chou, P.T.; Deng, C.; Chou, S.W.; Yuan, Z.; Liu, T.M. Low-toxicity FePt nanoparticles for the targeted and enhanced diagnosis of breast tumors using few centimeters deep whole-body photoacoustic imaging. Photoacoustics 2020, 19, 100179.
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115.
- Rehbock, C.; Barcikowski, S. Toxicity of Colloidal Alloy Nanoparticles. In Nanoalloys; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–449.
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112.
- Yamanaka, S. A fresh look at iPS cells. Cell 2009, 137, 13–17.
- Malgieri, A.; Kantzari, E.; Patrizi, M.P.; Gambardella, S. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int. J. Clin. Exp. Med. 2010, 3, 248.
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228.
- Kerativitayanan, P.; Carrow, J.K.; Gaharwar, A.K. Nanomaterials for engineering stem cell responses. Adv. Healthc. Mater. 2015, 4, 1600–1627.
- Cha, C.; Liechty, W.B.; Khademhosseini, A.; Peppas, N.A. Designing biomaterials to direct stem cell fate. ACS Nano 2012, 6, 9353–9358.
- Wei, M.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75.
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074.
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986.
- Zhou, X.; Yuan, L.; Wu, C.; Luo, G.; Deng, J.; Mao, Z. Recent review of the effect of nanomaterials on stem cells. RSC Adv. 2018, 8, 17656–17676.
- Yi, D.K.; Nanda, S.S.; Kim, K.; Selvan, S.T. Recent progress in nanotechnology for stem cell differentiation, labeling, tracking and therapy. J. Mater. Chem. B 2017, 5, 9429–9451.
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264.
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifacio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213.
- Melo, M.A., Jr.; Santos, L.S.S.; Gonçalves, M.D.C.; Nogueira, A.F. Preparation of silver and gold nanoparticles: A simple method to introduce nanotechnology into teaching laboratories. Quím. Nova 2012, 35, 1872–1878.
- Iravani, S.; Thota, S.; Crans, D.C. Methods for Preparation of Metal Nanoparticles; Wiley: Weinheim, Germany, 2017; Volume 63, pp. 15–31.
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262.
- Kumar, M.; Varshney, L.; Francis, S. Radiolytic formation of Ag clusters in aqueous polyvinyl alcohol solution and hydrogel matrix. Radiat. Phys. Chem. 2005, 73, 21–27.
- Constantin, C.; Neagu, M.; Ion, R.M.; Gherghiceanu, M.; Stavaru, C. Fullerene–porphyrin nanostructures in photodynamic therapy. Nanomedicine 2010, 5, 307–317.
- Nanda, S.S.; Wang, T.; Yoon, H.Y.; An, S.S.A.; Hembram, K.P.S.S.; Kim, K.; Yi, D.K. Enhanced proliferation of rabbit chondrocytes by using a well circulated nanoshock system. Sci. Rep. 2021, 11, 19388.
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171.
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321.
- Lin, J.; Miao, L.; Zhong, G.; Lin, C.H.; Dargazangy, R.; Alexander-Katz, A. Understanding the synergistic effect of physicochemical properties of nanoparticles and their cellular entry pathways. Commun. Biol. 2020, 3, 205.
- Aithal, S.; Aithal, P.S. Green and eco-friendly Nanotechnology–concepts and industrial prospects. Int. J. Manag. Technol. Soc. Sci. 2021, 6, 1–31.
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792.
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Green nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 145–198.
- Puja, P.; Kumar, P. A perspective on biogenic synthesis of platinum nanoparticles and their biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 94–99.
- Zhao, X.; Zhou, L.; Riaz Rajoka, M.S.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835.
- Taherzadeh, M.J.; Fox, M.; Hjorth, H.; Edebo, L. Production of mycelium biomass and ethanol from paper pulp sulfite liquor by Rhizopus oryzae. Bioresour. Technol. 2003, 88, 167–177.
- Nayak, S.S.; Wadhawa, G.C.; Pathade, K.B.; Shivankar, V.S.; Mirgane, N.A. Green synthesis of the plant assisted nanoparticles from Euphorbia neriifolia L. and its application in the degradation of dyes from industrial waste. Plant Sci. Today 2021, 8, 380–385.
- Bairwa, P.; Devra, V. Experimental Investigation on Green Synthesis of Bimetallic Nanoparticles by Using Plant Extract: A Review. J. Nanoworld 2022, 8, 6–18.
- Selim, A.A.; Sakr, T.M.; Essa, B.M. Gold Nanoparticles: Synthesis, Functionalization and Biomedical Applications Especially in Cardiovascular Therapy. Pharm. Chem. J. 2023, 59, 29–39.
- Ogidi, C.O.; Emmanuel, O.P.; Daramola, O.O.; Bamigboye, O.; Malomo, O. Synthesis of Silver Nanoparticles using Cellulose and Starch Extracted from Brewer Spent Grain: Assessment of their Antimicrobial and Preservatives Activities. Turk. J. Agric. Food Sci. Technol. 2023, 11, 227–238.
This entry is offline, you can click here to edit this entry!
