Neglected tropical diseases (NTDs) are a significant global health problem. Additionally, anti-protozoan treatments are toxic, and their therapeutic regimens require prolonged treatment times and high concentrations of the drugs. Additionally, multi-resistant protozoan strains represent an important global emergency that must be addressed. For these reasons, global efforts are being made to identify new drug candidates that are capable of combating these kinds of diseases. This systematic review shows that 5-nitroimidazole derivatives have been successfully used against neglected tropical protozoan diseases (NTPDs), with a specific focus on three diseases: malaria, leishmaniasis, and human trypanosomiasis. Some nitroimidazole derivatives have been repurposed, and an important group of new drugs is available for the treatment of NTPDs.
- anti-protozoan treatments
- 5-nitroimidazole
- neglected diseases
- Leishmania
- Trypanosoma
- Plasmodium
1. Introduction
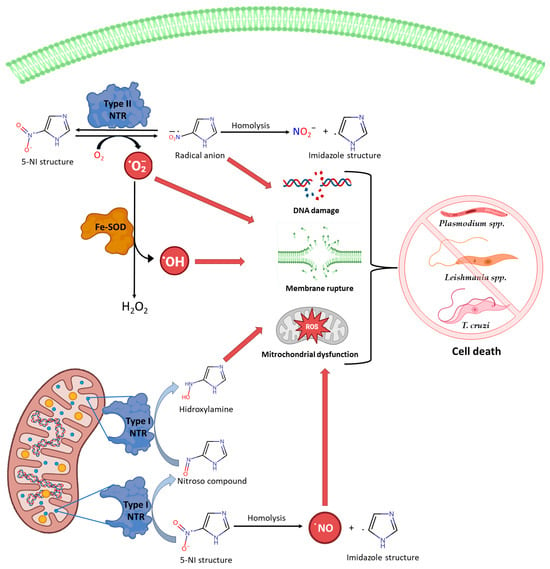
2. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/futurepharmacol4010015
References
- Lauwaet, T.; Miyamoto, Y.; Ihara, S.; Le, C.; Kalisiak, J.; Korthals, K.A.; Ghassemian, M.; Smith, D.K.; Sharpless, K.B.; Fokin, V.V.; et al. Click chemistry-facilitated comprehensive identification of proteins adducted by antimicrobial 5-nitroimidazoles for discovery of alternative drug targets against giardiasis. PLoS Negl. Trop. Dis. 2020, 14, e0008224.
- Martín-Escolano, R.; Pérez-Cordón, G.; Arán, V.J.; Marín, C.; Sánchez-Moreno, M.; Rosales, M.J. 5-Nitroindazole derivatives as potential therapeutic alternatives against Acanthamoeba castellanii. Acta Trop. 2022, 232, 106538.
- Borba, J.V.V.B.; Silva, A.C.; Lima, M.N.N.; Mendonca, S.S.; Furnham, N.; Costa, F.T.M.; Andrade, C.H. Chemogenomics and bioinformatics approaches for prioritizing kinases as drug targets for neglected tropical diseases. In Advances in Protein Chemistry and Structural Biology, 1st ed.; Rossen, D., Ed.; Elsevier: San Diego, CA, USA, 2021; Volume 124, pp. 187–223.
- Gupta, R.; Sharma, S.; Singh, R.; Vishwakarma, R.A.; Mignani, S.; Singh, P.P. Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals 2022, 15, 561.
- da Silva Santos-Júnior, P.F.; Rocha Silva, L.; Quintans-Júnior, L.J.; Ferreira da Silva-Júnior, E. Nitro compounds against trypanosomatidae parasites: Heroes or villains? Bioorg. Med. Chem. Lett. 2022, 75, 128930.
- Schmid, A.; Schmid, H. Pharmaco-toxicological mode of action of antimicrobial 5-nitroimidazole derivatives. J. Vet. Med. A Physiol. Pathol. Clin. Med. 1999, 46, 517–522.
- Graves, K.J.; Novak, J.; Secor, W.E.; Kissinger, P.J.; Schwebke, J.R.; Muzny, C.A. A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis. Parasitology 2020, 147, 1383–1391.
- Pasupuleti, V.; Escobedo, A.A.; Deshpande, A.; Thota, P.; Roman, Y.; Hernandez, A.V. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: A systematic review of randomized controlled trials. PLoS Negl. Trop. Dis. 2014, 8, e2733.
- Aspatwar, A.; Parvathaneni, N.K.; Barker, H.; Anduran, E.; Supuran, C.T.; Dubois, L.; Lambin, P.; Parkkila, S.; Winum, J.Y. Design, synthesis, in vitro inhibition and toxicological evaluation of human carbonic anhydrases I, II and IX inhibitors in 5-nitroimidazole series. J. Enzym. Inhib. Med. Chem. 2020, 35, 109–117.
- Peerzada, M.N.; Khan, P.; Khan, N.S.; Avecilla, F.; Siddiqui, S.M.; Hassan, M.I.; Azam, A. Design and Development of Small-Molecule Arylaldoxime/5-Nitroimidazole Hybrids as Potent Inhibitors of MARK4: A Promising Approach for Target-Based Cancer Therapy. ACS Omega 2020, 5, 22759–22771.
- López-López, E.; Cerda-García-Rojas, C.M.; Medina-Franco, J.L. Tubulin Inhibitors: A Chemoinformatic Analysis Using Cell-Based Data. Molecules 2021, 26, 2483.
- Zou, M.; Duan, Y.; Wang, P.; Gao, R.; Chen, X.; Ou, Y.; Liang, M.; Wang, Z.; Yuan, Y.; Wang, L.; et al. DYT-40, a novel synthetic 2-styryl-5-nitroimidazole derivative, blocks malignant glioblastoma growth and invasion by inhibiting AEG-1 and NF-κB signaling pathways. Sci. Rep. 2016, 6, 27331.
- López-López, E.; Naveja, J.J.; Medina-Franco, J.L. DataWarrior: An evaluation of the open-source drug discovery tool. Expert. Opin. Drug Discov. 2019, 14, 335–341.
- Bajorath, J.; Chávez-Hernández, A.L.; Duran-Frigola, M.; Fernández-de Gortari, E.; Gasteiger, J.; López-López, E.; Maggiora, G.M.; Medina-Franco, J.L.; Méndez-Lucio, O.; Mestres, J.; et al. Chemoinformatics and artificial intelligence colloquium: Progress and challenges in developing bioactive compounds. J. Cheminform. 2022, 14, 82.
- Campos-Fernández, L.; Ortiz-Muñiz, R.; Cortés-Barberena, E.; Mares-Sámano, S.; Garduño-Juárez, R.; Soriano-Correa, C. Imidazole and nitroimidazole derivatives as NADH-fumarate reductase inhibitors: Density functional theory studies, homology modeling, and molecular docking. J. Comput. Chem. 2022, 43, 1573–1595.
- The Carcinogenic Potency Project (CPDB). Available online: https://files.toxplanet.com/cpdb/index.html (accessed on 18 December 2023).
- Eke, I.G.; Eze, I.O.; Ezeudu, T.A.; Eze, U.U.; Anaga, A.O.; Onyeyili, P.A. Anti-trypanosomal activity of secnidazole in vitro and in vivo. Trop. J. Pharm. Res. 2017, 16, 535.
- Oliveira, A.A.; Oliveira, A.P.A.; Franco, L.L.; Ferencs, M.O.; Ferreira, J.F.G.; Bachi, S.M.P.S.; Speziali, N.L.; Farias, L.M.; Magalhães, P.P.; Beraldo, H. 5-Nitroimidazole-derived Schiff bases and their copper(II) complexes exhibit potent antimicrobial activity against pathogenic anaerobic bacteria. Biometals 2018, 31, 571–584.
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473.
- Enanga, B.; Ndong, J.M.; Boudra, H.; Debrauwer, L.; Dubreuil, G.; Bouteille, B.; Chauvière, G.; Labat, C.; Dumas, M.; Périé, J.; et al. Pharmacokinetics, metabolism and excretion of megazol in a Trypanosoma brucei gambiense primate model of human African trypanosomiasis. Preliminary study. Arzneimittelforschung 2000, 50, 158–162.
- Galasse Rando, D.G.; de Oliveira Costa, H.G.; Fernanda Heitor, T.; de Moraes, J.; Amorim Pavani, T.F. Employing “red flags” to fight the most neglected diseases: Nitroaromatic as still suitable tools to treat human and veterinary parasitosis. Curr. Top. Med. Chem. 2023, 23, 816–832.
- Leitsch, D.; Burgess, A.G.; Dunn, L.A.; Krauer, K.G.; Tan, K.; Duchêne, M.; Upcroft, P.; Eckmann, L.; Upcroft, J.A. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 2011, 66, 1756–1765.
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.L.; Read, K.D.; Fairlamb, A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900.
- López-López, E.; Bajorath, J.; Medina-Franco, J.L. Informatics for chemistry, biology, and biomedical sciences. J. Chem. Inf. Model. 2021, 61, 26–35.
- Deng, S.; Chen, A.; Chen, W.; Lai, J.; Pei, Y.; Wen, J.; Yang, C.; Luo, J.; Zhang, J.; Lei, C.; et al. Fabrication of Biodegradable and Biocompatible Functional Polymers for Anti-Infection and Augmenting Wound Repair. Pharmaceuticals 2022, 15, 120.
- Jose, A.; Vijayan, V.; Sahadevan, R.; Porel, M.; Sadhukhan, S. Selective luminescent detection of 5-nitroimidazole antibiotics through self-aggregates of a single non-aromatic amino acid, L-lysine. Microchem. J. 2023, 197, 109802.
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Proc. Eng. 2022, 45, 102474.
