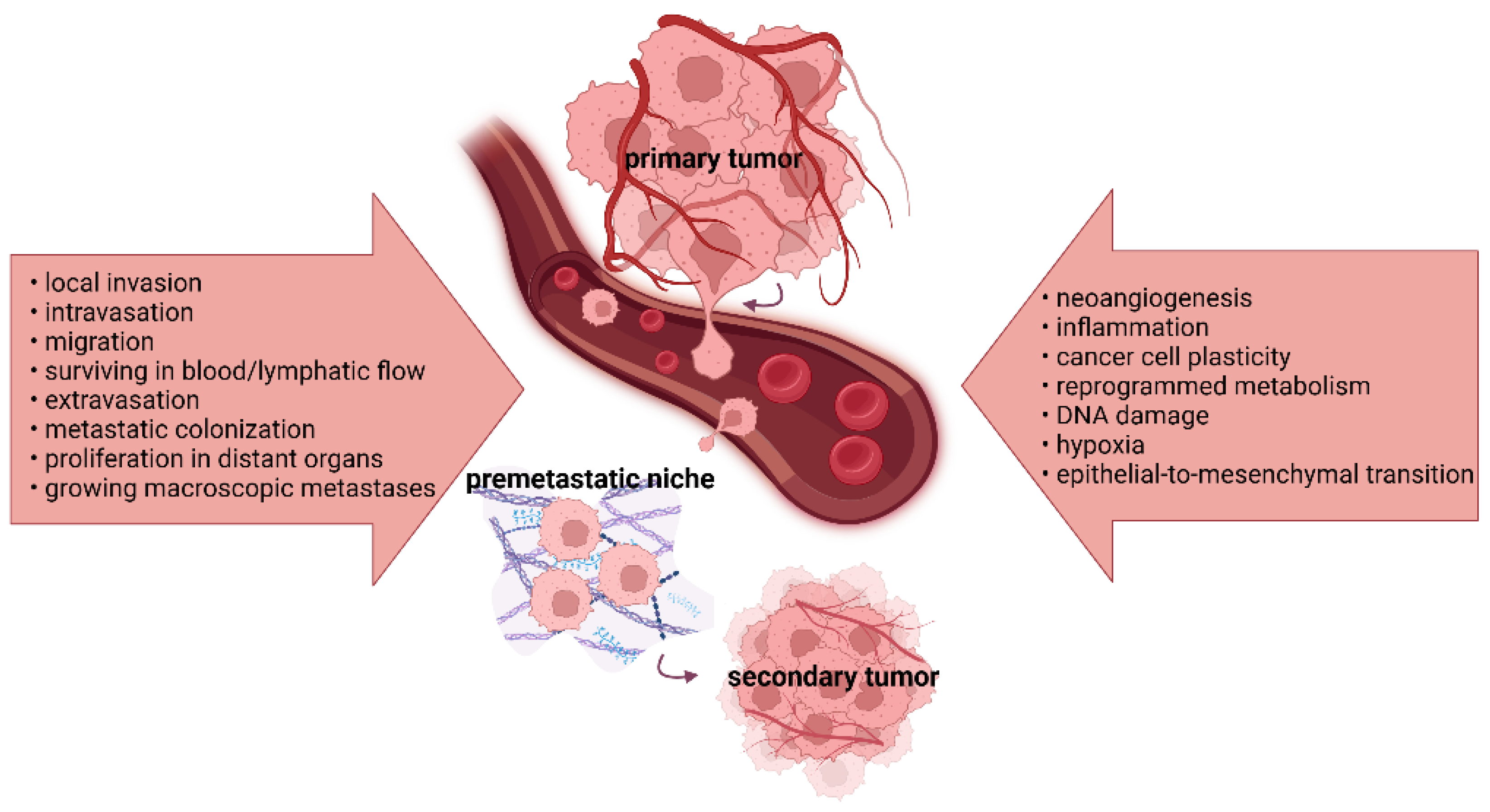
Cancer cell dissemination involves invasion, migration, resistance to stressors in the circulation, extravasation, colonization, and other functions responsible for macroscopic metastases. By enhancing invasiveness, motility, and intravasation, the epithelial-to-mesenchymal transition (EMT) process promotes the generation of circulating tumor cells and their collective migration. Preclinical and clinical studies have documented intensive crosstalk between the gut microbiome, host organism, and immune system. According to the findings, polymorphic microbes might play diverse roles in tumorigenesis, cancer progression, and therapy response. Microbial imbalances and changes in the levels of bacterial metabolites and toxins promote cancer progression via EMT and angiogenesis. In contrast, a favorable microbial composition, together with microbiota-derived metabolites, such as short-chain fatty acids (SCFAs), can attenuate the processes of tumor initiation, disease progression, and the formation of distant metastases.
- gut microbiome
- intratumoral microbiota
- cancer progression
- metastasis
- epithelial-to-mesenchymal transition
- angiogenesis
- microbiota modulation
1. Introduction
2. The Mechanisms of Tumor Progression and Metastasis
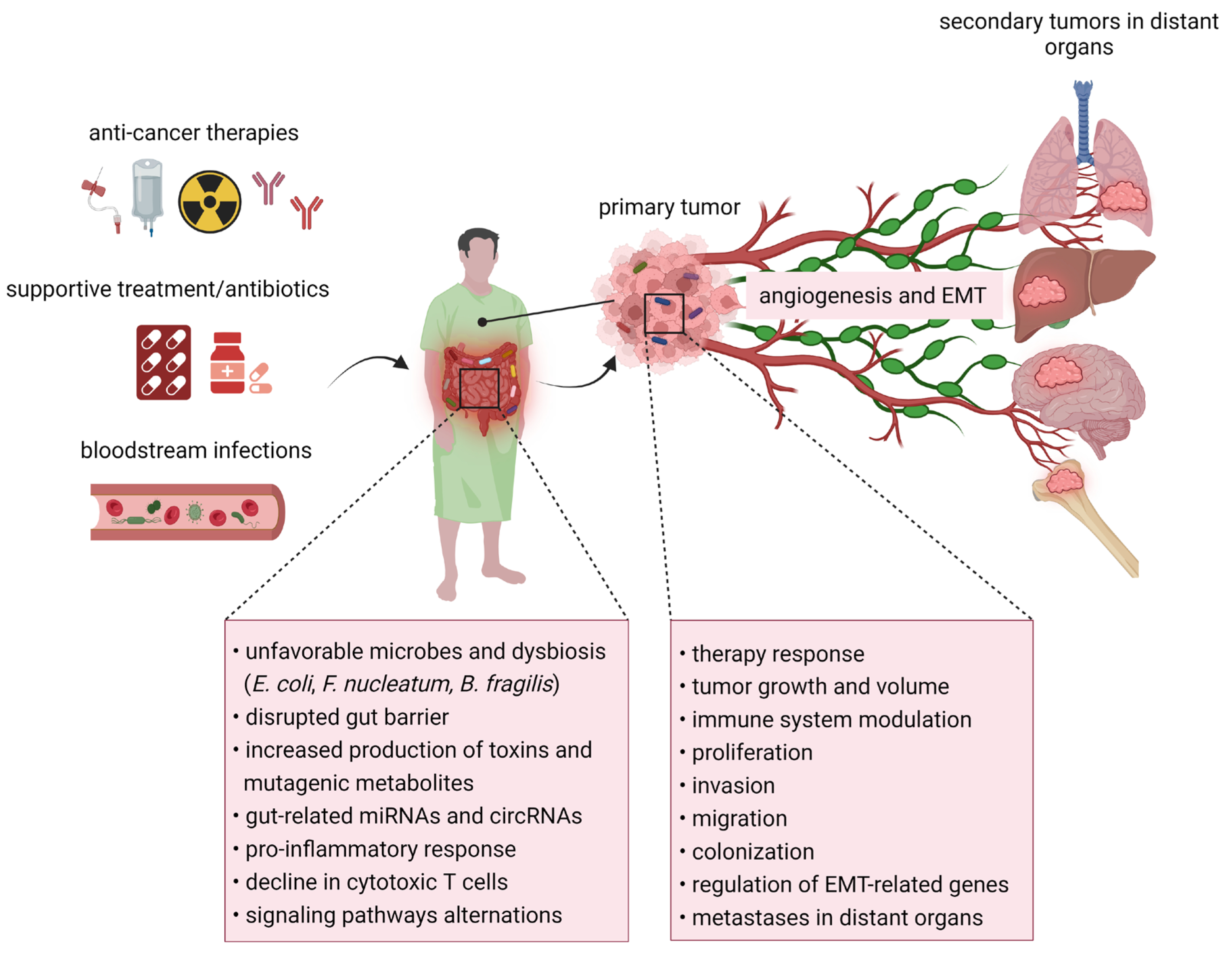
3. The Relationship between Microbiome and Cancer Progression-Related Processes
|
Study |
Study Design |
Disease |
Purpose |
Patients (n) |
Intervention |
Study Status |
|---|---|---|---|---|---|---|
|
NCT03941080 |
An observational prospective study |
Metastatic CRC |
To confirm the microbial taxa associated with treatment response and side effects in metastatic or irresectable disease |
300 adults/ older adults |
Enrolled patients will be newly diagnosed with an indication for standard palliative systemic treatment. |
Recruiting |
|
NCT04579484 |
An observational prospective study |
Metastatic breast cancer |
To determine the gut microbiome in fecal samples of patients with ER+ HER2− breast cancer and assess the relationship between dietary factors and microbiome |
20 adults/ older adults |
Patients will receive endocrine therapy with an aromatase inhibitor combined with an inhibitor of cyclin-dependent kinases 4 and 6. |
Recruiting |
|
NCT04804956 |
An observational prospective study |
Metastatic rectal cancer |
To identify the profile of the mesorectal microbiome and correlation with poor prognosis prediction |
100 adults |
Participants will receive neoadjuvant treatment. |
Recruiting |
|
NCT04579978 |
An observational prospective study |
Metastatic solid cancer |
To study changes in the gut microbial community after ICI and evaluate bacterial species associated with treatment efficacy |
60 adults/ older adults |
Patients will be enrolled in the study for planned standard-of-care ICI. |
Recruiting |
|
NCT05878977 |
An interventional open-label study |
Metastatic melanoma |
To define novel markers for the prediction of therapy response |
150 adults/ older adults |
Immunotherapy will consist of PD-1 and CTLA-4 inhibitors. |
Recruiting |
|
NCT05635149 |
An observational prospective study |
Metastatic CRC |
To assess the composition of the gut microbiome and its association with treatment efficacy |
100 adults/ older adults |
Patients will be treated with Fruquintinib, ICI plus RT, or Fruquintinib and ICI alone. |
Recruiting |
|
NCT05753839 |
An interventional randomized open-label study with parallel assignment |
Metastatic clear cell renal cell carcinoma/kidney cancer |
To correlate the gut and urine microbiome compositions with OS, PFS, and ORR |
40 adults/ older adults |
Patients will receive ICI followed by maintenance therapy with ICI or cytoreductive nephrectomy ± metastasectomy after ICI. |
Recruiting |
|
NCT04090710 |
An interventional randomized study with parallel assignment |
Metastatic renal cell carcinoma |
To investigate the changes in the gut microbiome via analysis of stool samples |
78 children/ adults/ older adults |
Patients will undergo cytoreductive stereotactic body RT with a combination of ICIs vs. one ICI alone. |
Recruiting |
|
NCT04243720 |
An observational prospective study |
Metastatic solid cancer |
To determine changes in the gut microbiome associated with resistance to immunotherapy |
100 adults/ older adults |
Only participants who progressed on immunotherapy will be enrolled in this study. |
Recruiting |
|
NCT04148378 |
An observational case-only prospective study |
CRC neoplasms/ metastatic CRC/ colorectal sarcoma/ adenocarcinoma |
To correlate microbiome composition with type of disease |
100 children/ adults/ older adults |
There is no intervention for the study. |
Unknown |
|
NCT04516135 |
An interventional randomized open-label study with parallel assignment |
Metastatic gynecologic cancers |
To describe overall diversity, richness, and specific microbial dynamics in the gut and vaginal microbiomes |
108 adults/ older adults |
Females will be treated with 3D conformal RT/intensity-modulated RT/volume-modulated arc therapy at the physician’s discretion for 1 fraction in the absence of RT-induced toxicities or progression. |
Recruiting |
|
NCT04214015 |
An observational case-only prospective study |
Metastatic mesothelioma |
To analyze the relative abundance of bacterial members in the gut microbiome |
100 children/ adults/ older adults |
There is no intervention for the study. |
Unknown |
|
NCT03818061 |
An interventional non-randomized study with parallel assignment |
Metastatic HNSCC |
To characterize the gut microbiome in immunotherapy using whole-metagenome sequencing |
33 adults/ older adults |
Patients with/without human papillomavirus will receive atezolizumab combined with bevacizumab. |
Active, but not recruiting |
|
NCT03698461 |
An interventional open-label study with single-group assignment |
Metastatic neoplasms/ colorectal neoplasms/ colonic neoplasms/ rectal neoplasms |
To determine fecal microbial profile in different time frames |
20 adults/ older adults |
Anti-cancer treatment will consist of atezolizumab with bevacizumab, levoleucovorin, oxaliplatin, and 5-fluorouracil. |
Active, but not recruiting |
|
NCT03977571 |
An interventional randomized open-label study with parallel assignment |
Metastatic renal cell carcinoma/ kidney cancer/ synchronous neoplasm |
To correlate the gut microbiome with OS, PFS, and ORR |
400 adults/ older adults |
Patients will receive deferred cytoreductive nephrectomy/no surgery following nivolumab with ipilimumab or tyrosine kinase inhibitors. |
Recruiting |
|
NCT04636775 |
An observational prospective study |
Metastatic non-small-cell lung cancer |
To assess the correlations between gut microbiome composition and adverse effects and differences between responders and non-responders |
46 adults/ older adults |
Patients will be treated with immunotherapy using ICI. |
Recruiting |
|
NCT04219137 |
An observational prospective study |
Metastatic EGA |
To study the microbiome in feces and rectal swab samples |
120 adults/ older adults |
Participants will undergo platinum-based chemotherapy. |
Unknown |
|
NCT03161756 |
An interventional non-randomized study parallel assignment |
Metastatic melanoma |
To explore associations between the gut microbiome and therapy response |
72 adults/ older adults |
Nivolumab alone or in combination with ipilimumab will be administered intravenously plus denosumab subcutaneously. |
Active, but not recruiting |
|
NCT04720768 |
An interventional open-label study with sequential assignment |
Metastatic melanoma |
To identify fecal biomarkers associated with therapy response/resistance |
78 adults/ older adults |
Patients will receive combined treatment with encorafenib, binimetinib, and palbociclib. |
Recruiting |
|
NCT03340129 |
An interventional randomized open-label study with parallel assignment |
Metastatic melanoma |
To observe the diversity and composition of the gut microbiome and to determine the correlation between mucosal integrity and microbes |
218 adults/ older adults |
Treatment will include ipilimumab and nivolumab with concurrent intracranial stereotactic RT or ipilimumab and nivolumab alone. |
Recruiting |
Abbreviations: CRC, colorectal cancer; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGA, esophagogastric adenocarcinoma; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitors; ORR, overall response rate; OS, overall survival; PD-1, programed cell death protein 1; PFS, progression-free survival; RT, radiotherapy.
4. The Studies of Microbiome Composition in Metastatic Disease
|
Malignancy |
Study Type Preclinical/Clinical |
Intervention |
Changes in Microbial Composition |
Major Findings |
Ref. |
|---|---|---|---|---|---|
|
CRC with liver/lung metastases |
Patients |
Regorafenib plus toripalimab |
Fusobacterium, Alistipes, Bilophila, and Acidaminococcus |
A higher level of specific bacteria was observed in non-responders. Shorter PFS correlated with a higher amount of Fusobacterium. |
[39] |
|
FAP |
Patients/ mice |
No intervention provided |
E. coli and ETBF |
Both bacterial taxa were biofilm members in FAP tissues from patients. Colonization with E. coli and ETBF increased DNA damage and IL-17 production in carcinogen-treated mice. |
[40] |
|
CRC with liver/lung metastases |
Patients |
Quxie capsules |
Actinobacteria, Oscillibacter, Eubacterium, and Lachnospiraceae |
Capsules increased butyrate-producing, immunity-stimulating, and anti-cancer bacterial taxa and enhanced Th cells, both CD4 and CD8 cells. |
[41] |
|
PDAC with lymph node metastases |
Patients |
No intervention |
Leuconostoc, Sutterella, Comamonas, and Turicibacter |
Lower levels of Leuconostoc and Sutterella were documented in tumors with a size ≥3 cm. An increase in lymph node metastases correlated with a higher abundance of Comamonas and Turicibacter. On the contrary, Streptococcus dominated recurrence-free tumors. |
[42] |
|
Hepatocellular carcinoma |
Mice |
NpRg3 |
Bacteroidetes, Verrucomicrobia, and Firmicutes |
Developed NpRg3 remodeled gut microbiome via reduced Firmicutes and increased Bacteroidetes and Verrucomicrobia in stool samples. Moreover, NpRg3 attenuated tumor development and lung metastatic formation in dimethylnitrosamine-induced spontaneous murine carcinoma. |
[43] |
|
Lung cancer |
Patients |
Systemic therapy/surgical resection |
Legionella and Thermus |
Thermus was abundant in the lung microbiome in patients with advanced cancer stages, while Legionella was enriched in patients with developed metastases. Alpha diversity in tumor tissues was lower than in non-malignant lung tissue samples. |
[44] |
|
Hormone receptor-positive breast cancer |
Mice |
Antibiotic cocktail (vancomycin, ampicillin, metronidazole, neomycin, and gentamicin) |
Blautia, Alistipes, Blautia, Escherichia/Shigella, and Bilophila |
Orally gavaged antibiotics caused commensal dysbiosis with a higher abundance of specific genera in poorly metastatic mice. Antibiotics promoted tumor cell dissemination to the lungs/peripheral blood/and lymph nodes. |
[45] |
|
Breast cancer |
Patients |
No intervention |
Streptococcus, Campylobacter, Moraxellaceae, Lactobacillales, Bacilli, Epsilonproteobacteria, Veillonella, Acinetobacter, Pseudomonadales, Megamonas, and Akkermansia |
Listed bacteria, except for Megamonas and Akkermansia, were increased in stool samples of patients with bone metastases. However, the results showed lowered levels of Megamonas and Akkermansia. Bacterial diversity was reduced in the order of normal controls, patients without metastases, and patients with bone metastases. |
[46] |
|
Breast cancer |
Patients |
Neoadjuvant chemotherapy |
Streptococcus, Pseudomonas, Brevundimonas, and Staphylococcus |
Chemotherapy decreased intratumoral Streptococcus and increased Pseudomonas. The development of distant metastases correlated with a higher presence of Brevundimonas and Staphylococcus in primary breast tumors. |
[47] |
|
Oral squamous cell carcinoma |
Patients |
Therapeutic neck dissection due to positive lymph node metastases |
Tannerella, Fusobacterium, Prevotella, Stomatobaculum, Bifidobacterium, Finegoldia Peptostreptococcaceae, and Shuttleworthia |
Two taxa—Tannerella and Fusobacterium—were enriched in the oral microbiome of patients without metastases. Other genera from the listed panel increased in patients with developed lymph node metastases. Differences in alpha diversity between the oral microbiome of 2 analyzed groups were not significant. |
[48] |
|
Castrate-resistant prostate cancer |
Patients |
Immune checkpoint inhibitor (pembrolizumab) |
A. muciniphila, B. thetaiotaomicron, B. fragilis, and R. unassigned |
A. muciniphila was depleted in pembrolizumab responders, while other listed microbes were higher in responding patients. |
[49] |
|
Renal cell carcinoma |
Patients |
Immune checkpoint inhibitor (nivolumab or nivolumab plus ipilimumab |
A. muciniphila, B. adolescentis, B. intestinihominis, Odoribacter splanchnicus, Bacteroides ovatus, and Eggerthella lenta |
A. muciniphila, B. adolescentis, B. intestinihominis, and O. splanchnicus correlated with clinical benefit in metastatic patients, while B. ovatus and E. lenta were associated with no clinical benefit from immunotherapy. |
[50] |
|
Renal cell carcinoma |
Patients |
Immune checkpoint inhibitor |
Akkermansia |
The presence of Akkermansia was documented in both responding and non-responding patients to immunotherapy. Therefore, host-specific or tumor factors might affect therapy response. |
[51] |
|
Melanoma |
Patients |
Immune checkpoint inhibitor |
Lactobacillales, Clostridiales/Ruminococcaceae, Faecalibacterium, Bacteroidales, B. thetaiotaomicron, E. coli, and Anaerotruncus colihominis |
Lactobacillales dominated the oral microbiome of all metastatic patients. Clostridiales/Ruminococcaceae, Faecalibacterium, and alpha diversity were greater in responders, while Bacteroidales, B. thetaiotaomicron, E. coli, and A. colihominis were abundant in non-responders. |
[52] |
|
Melanoma |
Patients |
Immune checkpoint inhibitor |
B. longum, C. aerofaciens, E. faecium, R. obeum, and R. intestinalis |
The authors observed a higher abundance of R. obeum and R. intestinalis in non-responders, while the other 3 species were enriched significantly in the responder gut microbiome. |
[53] |
|
Melanoma |
Patients |
Immune checkpoint inhibitor |
Clostridiales and Bacteroidales |
Higher bacterial diversity with the prevalence of Clostridiales was observed in the gut microbiome of responding patients. However, the dominance of Bacteroidales within the gut microbiome characterized non-responders. |
[54] |
|
Melanoma |
Patients |
No intervention |
Corynebacterium |
In swab samples, Corynebacterium was the most detected taxa in advanced-stage patients. However, the authors did not detect associations between cutaneous microbiome and cancer stage. |
[55] |
Abbreviations: ETBF, enterotoxigenic B. fragilis; FAP, familial adenomatous polyposis; NpRg3, nanoparticle conjugation of ginsenoside Rg3; PDAC, pancreatic ductal adenocarcinoma; PFS, progression-free survival; Th cells, T helper cells.
5. Microbiota Modulation and Cancer Progression
This entry is adapted from the peer-reviewed paper 10.3390/ijms242417199
References
- Sevcikova, A.; Izoldova, N.; Stevurkova, V.; Kasperova, B.; Chovanec, M.; Ciernikova, S.; Mego, M. The Impact of the Microbiome on Resistance to Cancer Treatment with Chemotherapeutic Agents and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 488.
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Massague, J.; Ganesh, K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021, 11, 971–994.
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579.
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28.
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454.
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352.
- Giuliano, M.; Giordano, A.; Jackson, S.; Hess, K.R.; De Giorgi, U.; Mego, M.; Handy, B.C.; Ueno, N.T.; Alvarez, R.H.; De Laurentiis, M.; et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011, 13, R67.
- Mego, M.; Karaba, M.; Minarik, G.; Benca, J.; Silvia, J.; Sedlackova, T.; Manasova, D.; Kalavska, K.; Pindak, D.; Cristofanilli, M.; et al. Circulating Tumor Cells with Epithelial-to-mesenchymal Transition Phenotypes Associated with Inferior Outcomes in Primary Breast Cancer. Anticancer Res. 2019, 39, 1829–1837.
- Fridrichova, I.; Kalinkova, L.; Ciernikova, S. Clinical Relevancy of Circulating Tumor Cells in Breast Cancer: Epithelial or Mesenchymal Characteristics, Single Cells or Clusters? Int. J. Mol. Sci. 2022, 23, 12141.
- Kim, M.Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.; Norton, L.; Massague, J. Tumor self-seeding by circulating cancer cells. Cell 2009, 139, 1315–1326.
- Comen, E.; Norton, L. Self-seeding in cancer. Recent Results Cancer Res. 2012, 195, 13–23.
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138.
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458.
- Zhong, W.; Wang, Q.; Shen, X.; Du, J. The emerging role of neutrophil extracellular traps in cancer: From lab to ward. Front. Oncol. 2023, 13, 1163802.
- Adrover, J.M.; McDowell, S.A.C.; He, X.Y.; Quail, D.F.; Egeblad, M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell 2023, 41, 505–526.
- Hu, W.; Lee, S.M.L.; Bazhin, A.V.; Guba, M.; Werner, J.; Niess, H. Neutrophil extracellular traps facilitate cancer metastasis: Cellular mechanisms and therapeutic strategies. J. Cancer Res. Clin. Oncol. 2023, 149, 2191–2210.
- Nepali, P.R.; Kyprianou, N. Anoikis in phenotypic reprogramming of the prostate tumor microenvironment. Front. Endocrinol. 2023, 14, 1160267.
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 3481–3498.
- Pretzsch, E.; Bosch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J. Oncol. 2019, 2019, 7407190.
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317.
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827.
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249.
- Kryczek, I.; Wei, S.; Keller, E.; Liu, R.; Zou, W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am. J. Physiol. Cell Physiol. 2007, 292, C987–C995.
- Xu, C.; Zhao, H.; Chen, H.; Yao, Q. CXCR4 in breast cancer: Oncogenic role and therapeutic targeting. Drug Des. Dev. Ther. 2015, 9, 4953–4964.
- Muller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56.
- Kaplan, R.N.; Rafii, S.; Lyden, D. Preparing the “soil”: The premetastatic niche. Cancer Res. 2006, 66, 11089–11093.
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474.
- Kim, H.J.; Ji, Y.R.; Lee, Y.M. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Arch. Pharm. Res. 2022, 45, 401–416.
- Zirlik, K.; Duyster, J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol. Res. Treat. 2018, 41, 166–171.
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266.
- Harry, J.A.; Ormiston, M.L. Novel Pathways for Targeting Tumor Angiogenesis in Metastatic Breast Cancer. Front. Oncol. 2021, 11, 772305.
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-Angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840.
- Hilmi, M.; Kamal, M.; Vacher, S.; Dupain, C.; Ibadioune, S.; Halladjian, M.; Sablin, M.P.; Marret, G.; Ajgal, Z.C.; Nijnikoff, M.; et al. Intratumoral microbiome is driven by metastatic site and associated with immune histopathological parameters: An ancillary study of the SHIVA clinical trial. Eur. J. Cancer 2023, 183, 152–161.
- Hajjar, J.; Mendoza, T.; Zhang, L.; Fu, S.; Piha-Paul, S.A.; Hong, D.S.; Janku, F.; Karp, D.D.; Ballhausen, A.; Gong, J.; et al. Associations between the gut microbiome and fatigue in cancer patients. Sci. Rep. 2021, 11, 5847.
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e36.
- Spakowicz, D.; Hoyd, R.; Muniak, M.; Husain, M.; Bassett, J.S.; Wang, L.; Tinoco, G.; Patel, S.H.; Burkart, J.; Miah, A.; et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: Causal modeling, timing, and classes of concomitant medications. BMC Cancer 2020, 20, 383.
- Wang, F.; He, M.M.; Yao, Y.C.; Zhao, X.; Wang, Z.Q.; Jin, Y.; Luo, H.Y.; Li, J.B.; Wang, F.H.; Qiu, M.Z.; et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: A phase Ib/II clinical trial and gut microbiome analysis. Cell Rep. Med. 2021, 2, 100383.
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597.
- Sun, L.; Yan, Y.; Chen, D.; Yang, Y. Quxie Capsule Modulating Gut Microbiome and Its Association with T cell Regulation in Patients with Metastatic Colorectal Cancer: Result From a Randomized Controlled Clinical Trial. Integr. Cancer Ther. 2020, 19, 1534735420969820.
- Jeong, J.Y.; Kim, T.B.; Kim, J.; Choi, H.W.; Kim, E.J.; Yoo, H.J.; Lee, S.; Jun, H.R.; Yoo, W.; Kim, S.; et al. Diversity in the Extracellular Vesicle-Derived Microbiome of Tissues according to Tumor Progression in Pancreatic Cancer. Cancers 2020, 12, 2346.
- Ren, Z.; Chen, X.; Hong, L.; Zhao, X.; Cui, G.; Li, A.; Liu, Y.; Zhou, L.; Sun, R.; Shen, S.; et al. Nanoparticle Conjugation of Ginsenoside Rg3 Inhibits Hepatocellular Carcinoma Development and Metastasis. Small 2020, 16, e1905233.
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016, 17, 163.
- Buchta Rosean, C.; Bostic, R.R.; Ferey, J.C.M.; Feng, T.Y.; Azar, F.N.; Tung, K.S.; Dozmorov, M.G.; Smirnova, E.; Bos, P.D.; Rutkowski, M.R. Preexisting Commensal Dysbiosis Is a Host-Intrinsic Regulator of Tissue Inflammation and Tumor Cell Dissemination in Hormone Receptor-Positive Breast Cancer. Cancer Res. 2019, 79, 3662–3675.
- Wenhui, Y.; Zhongyu, X.; Kai, C.; Zhaopeng, C.; Jinteng, L.; Mengjun, M.; Zepeng, S.; Yunshu, C.; Peng, W.; Yanfeng, W.; et al. Variations in the Gut Microbiota in Breast Cancer Occurrence and Bone Metastasis. Front. Microbiol. 2022, 13, 894283.
- Chiba, A.; Bawaneh, A.; Velazquez, C.; Clear, K.Y.J.; Wilson, A.S.; Howard-McNatt, M.; Levine, E.A.; Levi-Polyachenko, N.; Yates-Alston, S.A.; Diggle, S.P.; et al. Neoadjuvant Chemotherapy Shifts Breast Tumor Microbiota Populations to Regulate Drug Responsiveness and the Development of Metastasis. Mol. Cancer Res. 2020, 18, 130–139.
- Eun, Y.G.; Lee, J.W.; Kim, S.W.; Hyun, D.W.; Bae, J.W.; Lee, Y.C. Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Sci. Rep. 2021, 11, 23176.
- Peiffer, L.B.; White, J.R.; Jones, C.B.; Slottke, R.E.; Ernst, S.E.; Moran, A.E.; Graff, J.N.; Sfanos, K.S. Composition of gastrointestinal microbiota in association with treatment response in individuals with metastatic castrate resistant prostate cancer progressing on enzalutamide and initiating treatment with anti-PD-1 (pembrolizumab). Neoplasia 2022, 32, 100822.
- Salgia, N.J.; Bergerot, P.G.; Maia, M.C.; Dizman, N.; Hsu, J.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti-PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 498–502.
- Agarwal, A.; Modliszewski, J.; Davey, L.; Reyes-Martinez, M.; Runyambo, D.; Corcoran, D.; Dressman, H.; George, D.J.; Valdivia, R.H.; Armstrong, A.J.; et al. Investigating the role of the gastrointestinal microbiome in response to immune checkpoint inhibitors (ICIs) among patients (pts) with metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 2020, 38, 730.
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103.
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108.
- Wargo, J.A.; Gopalakrishnan, V.; Spencer, C.; Karpinets, T.; Reuben, A.; Andrews, M.C.; Tetzlaff, M.T.; Lazar, A.; Hwu, P.; Hwu, W.J.; et al. Association of the diversity and composition of the gut microbiome with responses and survival (PFS) in metastatic melanoma (MM) patients (pts) on anti-PD-1 therapy. J. Clin. Oncol. 2017, 15, 3008.
- Mizuhashi, S.; Kajihara, I.; Sawamura, S.; Kanemaru, H.; Makino, K.; Aoi, J.; Makino, T.; Masuguchi, S.; Fukushima, S.; Ihn, H. Skin microbiome in acral melanoma: Corynebacterium is associated with advanced melanoma. J. Dermatol. 2021, 48, e15–e16.
- Ciernikova, S.; Mego, M.; Hainova, K.; Adamcikova, Z.; Stevurkova, V.; Zajac, V. Modification of microflora imbalance: Future directions for prevention and treatment of colorectal cancer? Neoplasma 2015, 62, 345–352.
- Wang, Z.; Li, L.; Wang, S.; Wei, J.; Qu, L.; Pan, L.; Xu, K. The role of the gut microbiota and probiotics associated with microbial metabolisms in cancer prevention and therapy. Front. Pharmacol. 2022, 13, 1025860.
- Luo, M.; Hu, M.; Xu, F.; Wu, X.; Dong, D.; Wang, W. Preventive effect of Lactobacillus reuteri on melanoma. Biomed. Pharmacother. 2020, 126, 109929.
- Pereira, F.V.; Melo, A.C.L.; Silva, M.B.; de Melo, F.M.; Terra, F.F.; Castro, I.A.; Perandini, L.A.; Miyagi, M.T.; Sato, F.T.; Origassa, C.S.T.; et al. Interleukin-6 and the Gut Microbiota Influence Melanoma Progression in Obese Mice. Nutr. Cancer 2021, 73, 642–651.
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and Butyrate Produced by Gut Microbiota after Probiotic Supplementation Attenuate Lung Metastasis of Melanoma Cells in Mice. Mol. Nutr. Food Res. 2021, 65, e2100096.
- Le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote Immunosurveillance against Lung Metastases. Cell Rep. 2018, 24, 3528–3538.
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamaki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315.
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712.
- Jakubauskas, M.; Jakubauskiene, L.; Leber, B.; Horvath, A.; Strupas, K.; Stiegler, P.; Schemmer, P. Probiotic Supplementation Attenuates Chemotherapy-Induced Intestinal Mucositis in an Experimental Colorectal Cancer Liver Metastasis Rat Model. Nutrients 2023, 15, 1117.
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224–1232.
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609.
