Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Cyclodextrin-based nanosponges (CDNSs) are complex macromolecular structures composed of individual cyclodextrins (CDs) and nanochannels created between cross-linked CD units and cross-linkers.
- cyclodextrin
- nanosponge
- drug delivery system
- synthesis
- physicochemical
1. Introduction
The European Medicines Agency (EMA) distinguishes almost 600 different types of dosage forms in standard use, a large share of which are oral medications [1]. According to financial reports, oral formulations contributed to about 42.2% of the drug market share in 2021, of which 53.1% was held by tablets alone [2][3]. Pharmaceuticals prepared for oral intake are convenient to use, but their activity is disturbed due to diverse processes of drug disposition in the organism—absorption, distribution, metabolism, and excretion (ADME). Since the efficiency of the drugs is dose-dependent, absorption is usually named as the most influential part of the metabolic journey of orally administered pharmaceuticals. In the case of extremely poor oral intake characteristics, e.g., instability in gastrointestinal environment or intestinal wall permeability limitations due to the structural properties or electric charge of the drug, a new route of administration should be considered. No drug presents perfect pharmacokinetic parameters in oral use; thus, the formulation development in the pharmaceutical industry is still valid.
Knowing that the drug displays a suitable pharmacodynamic effect, research on improving bioavailability and reducing adverse effects is required. These objectives can be achieved by chemical modification of the drug itself (e.g., the formation of prodrugs or better-absorbed drug forms) or by implementation of a pharmaceutical into the transport systems. Finding the optimal drug delivery system for a given drug is difficult, due to a series of factors, like complex stability, the toxicity of the formulation, or the influence on the drug’s pharmacokinetic parameters or synthesis costs, which are crucial for proper therapeutic effectiveness and optimization of the technological processes of drug formulations’ production.
The last 50 years have witnessed the evolution of drug manufacturing, caused by implementation of numerous new formulating agents. As the 21st century arrived, a vigorously developing branch of nanotechnology arose, exerting a major influence on electronics, and also being used in biological applications. In the last 15 years, nanosponge particles (named for their sponge-like structure) have been intensively studied due to their possible applications, e.g., as drug carriers, toxin removal agents, or catalysts in organic syntheses [4][5][6][7][8].
2. Cyclodextrin-Based Drug Delivery Systems—A Short Summary
2.1. Cyclodextrins
Cyclodextrins (hereinafter referred to as CDs) are cyclic oligosaccharides composed of α-d-glucopyranose units combined with α-(1→4)-glycosidic bonds. Native CDs are a product of the enzymatic degradation of starch, in which cyclodextrin glycosyltransferases produce cyclic oligomers consisting of six (α-CD), seven (β-CD, Figure 2a), or eight (γ-CD) glucose units [9][10][11][12]. Due to restricted rotation of glycosidic bonds and the chair conformation of each glucose unit, CDs possess the specific shape of a truncated cone with a hollow cavity (Figure 2b) [11][12][13][14][15].
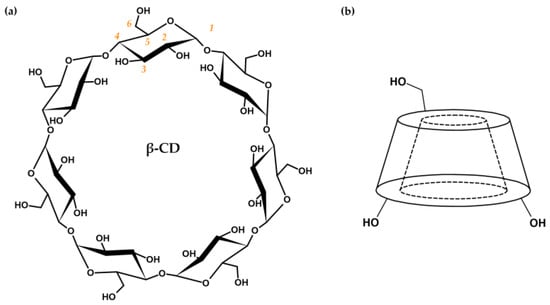
Figure 2. (a) Cyclodextrin structure based on β-CD with glucose units in chair conformation. The numbering order of carbon atoms of the glucose subunit is marked in orange. (b) Schematic representation of the CD shape, with labeled primary and secondary hydroxyl groups on the outer surfaces of the narrower and wider edges, respectively.
The inner surface of CDs is lined with skeletal C-H groups and ethereal oxygen atoms that contribute to the lipophilic character of the cavity. The outer surface is composed of primary (at C6, narrower edge) and secondary (at C2 and C3, wider edge) hydroxyl groups that are responsible for the hydrophilic features of CDs (Figure 2b). Amphiphilic properties enable the binding of lipophilic drugs inside the CD cavities in the form of host–guest inclusion complexes [16][17][18][19]. Complexation of the drug and stabilization of the obtained complex are the result of conformational changes in the CD structure (steric relaxation of the ring) and the formation of various non-covalent bonds, i.e., van der Waals, hydrophobic, dipole–dipole, electrostatic interactions, hydrogen bonds, or dispersion forces [9][20][21][22][23]. On the other hand, the hydrophilic outer surface is responsible for increasing drug solubility through interactions with aqueous media via hydroxyl groups. Thus, CDs are great candidates for drug transport systems [9][11][12][13][14][19][20][24].
2.2. Cyclodextrin-Based Nanosponges
In addition to enhancing solubility and bioavailability, CDs have several drawbacks. Based on cavity size, CDs are usually capable of binding only one drug molecule (fully or partially), showing poor drug loading capacity. Additionally, relatively low stability constants of drug–CD complexes contribute to easy dissociation and release of their contents [9][14][16][25][26]. However, due to highly reactive hydroxyl groups, CDs can act as polyfunctional monomers [27][28]. Polymerization of CDs leads to the formation of cyclodextrin-based nanosponges (CDNSs) [16][29]. For CDNS synthesis, both natural and synthetic derivatives of cyclodextrins, e.g., 2-hydroxypropyl-β-CD (2-HP-β-CD), carboxymethyl-β-CD, sulfobutylether-β-CD, tosyl-β-CD, and a variety of methylated derivatives, can be used [13][18][19][30]. Nevertheless, β-CD is the most commonly used, owing to its non-toxic nature, low production costs, and highest stability constants in complexes with drugs [13][31][32][33]. Nanosponges are formed using linking agents called cross-linkers, which are highly reactive substances containing at least two active sites capable of covalent binding with hydroxyl groups of CDs. Cross-linking is a condensation polymerization reaction requiring the activation of CD hydroxyl groups by an electron-withdrawing group of the cross-linker. Activated hydroxyl groups are attacked with nucleophilic sites of the cross-linker, which usually binds with the primary hydroxyl group at C6 [4][28][34].
Nanosponge particles are spherical and have a maximum diameter of 1 μm. The three-dimensional structure of CDNSs consists of parent CDs with their lipophilic cavities and nanochannels created between cross-linked CD units and cross-linkers (Figure 3). The hydrophilic properties of nanochannels are the result of the presence of multiple hydrophilic moieties of cross-linker molecules and free hydroxyl groups of CDs. Amphiphilic properties enable CDNSs to encapsulate a variety of drugs, both hydrophilic and lipophilic, in the form of non-inclusion or inclusion complexes. The former are made due to drug absorption on the CDNS surface, and they then migrate into hydrophilic channels. The latter are created through diffusion of the drug through the CDNS structure and inside the CD cavity [35][36][37][38]. The obtained drug–CDNS complexes present one of the highest stability constants for known non-covalent complexes (approx. 108 M−1), much more stable in comparison with drug–CD complexes (50–2000 M−1) [39][40]. High stability could indicate the formation of irreversible complexes; however, in reality, the stability is disturbed in aqueous media, and the drug can be vigorously or gradually released from the CDNS. This phenomenon is the basis of the modifiable drug release properties of CDNSs, one of the most important properties of drug–CDNS complexes, which also reduces the likelihood of adverse effects [16]. Moreover, CDNSs are non-toxic, thermally stable up to 300 °C, chemically stable in environments of pH 1 to 11, and protect the drug against physical and chemical factors. Their production is simple, low-cost, and easy to scale up; they are compatible with most vehicles and excipients and are insoluble in most organic solvents and water, which is helpful in their purification and incorporation into CDNSs. As drug carriers, CDNSs can be formulated for oral, topical, parenteral, or inhalation use. Lastly, they are highly biodegradable—hydrolysis of CDNSs leads to parent CDs, which are completely degraded to glucose or maltodextrins by the colonic microflora. All of these properties make CDNSs versatile drug delivery systems with multiple possible medical applications [14][31][32][38][41][42][43][44][45][46].
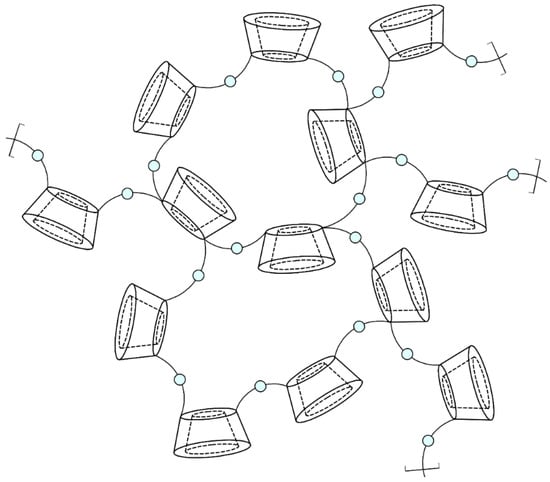
Figure 3. The structure of cyclodextrin-based nanosponges: Blue beads represent cross-linker molecules, which bind mostly to C6 hydroxyl groups on the narrower edge of the CD units (schematic structure, for visualization purposes only; degree of cross-linking not preserved).
However, further studies on using CDNSs as drug carriers should be performed, which, in turn, would earn the approval of the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for general use. At present, no drug–CDNS complex has received such approval. In 2023, there were 129 different formulations of drug–CD complexes recognized for medicinal general use, whereas ten years previously there were only 48 [47]. This looks promising in relation to CDNSs, which present better physicochemical properties required for good drug delivery systems as compared with CDs.
-
First generation: plain nanosponges (Figure 4)—CDs polymerized with a cross-linker, used to obtain higher generations of CDNSs; cross-linker choice subdivides the generation into four types:
- a.
-
Carbamate nanosponges—based on urethane compounds, e.g., hexamethylene diisocyanate (HMDI) or toluene-2,4-diisocyanate (TDI);
- b.
-
Carbonate nanosponges—based on active carbonyl compounds, e.g., diphenyl carbonate (DPC), dimethyl carbonate (DMC), or 1,1′-carbonyldiimidazole (CDI);
- c.
-
Ester nanosponges—based on di- or polycarboxylic acids or their dianhydrides, e.g., citric acid (CA), pyromellitic dianhydride (PMDA), or ethylenediaminetetraacetic dianhydride (EDTA);
- d.
-
Ether nanosponges—based on epoxide-bearing cross-linkers, e.g., epichlorohydrin (EPI); since EPI was found to be toxic to humans, safety concerns were raised about its use in nanosponge production; however, it was proven that EPI-based CDNSs are non-toxic, owing to the fast hydrolysis of free EPI into harmless products during CDNS degradation [48].
-
Second generation: modified nanosponges—equipped with chemical groups ensuring additional chemical or physical properties, such as fluorescence (ADME in vivo) or electric charge (binding polar drugs, increasing the stability of CDNS suspensions by inducing repulsive negative potential between particles).
-
Third generation: stimulus-responsive nanosponges—sensitive to environmental stimuli such as temperature, pH, or redox potential, which can alter their physicochemical properties to trigger or enhance the release of the drug.
-
Fourth generation: molecularly imprinted polymer nanosponges (MIP-NSs)—possessing binding sites with the imprinted structure of the drug, giving CDNSs high selectivity and affinity for target molecules.
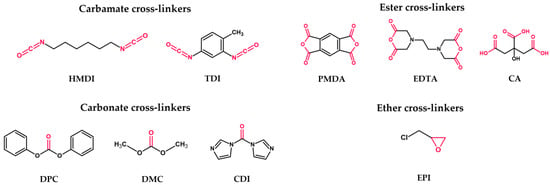
Figure 4. Chemical structures of the most frequently used cross-linkers. The donor moieties of each cross-linker group are labeled with red.
Krabicova et al. [49] highlighted the possibility of the development of a fifth CDNS generation that, due to grafting the biological ligands on the CDNS surface, would bind with specific molecular targets to improve the drug’s bioavailability and the therapeutic effect. Apart from drug carrier applications, CDNS properties have been applied to other medicinal uses, such as protein [50] and enzyme [51] delivery systems, taste-masking agents [52], biosensors [53][54], gas transporters [55][56][57], and in fabric functionalization procedures [58][59].
2.3. Methods of CDNS Synthesis
-
The melting method, during which CDs and cross-linkers are combined by melting at a temperature of up to 130 °C for several hours—longer synthesis results in nanosponges with a higher cross-linking degree;
-
The solvent method, in which CDs and cross-linkers are solubilized in organic solvents, usually N,N-dimethylformamide (DMF) or dimethyl sulfoxide (DMSO), the use of which is justified by solvation of CDNSs and drug release from CD in an aqueous environment; cross-linking is initiated at a temperature ranging from 10 °C to the reflux temperature of the solvent and can last for up to 48 h;
-
The ultrasound-assisted method, which uses ultrasound as a homogenizer in the presence of a solvent or in a solvent-free environment; the reaction proceeds at 90 °C for five hours in an ultrasonic bath filled with water;
-
The microwave-assisted method, where microwave irradiation plays the role of homogenizing agent, reducing the reaction time and ensuring proper homogenization of substrates.
Each cross-linker type requires a different synthesis approach. The use of dianhydrides requires the addition of triethylamine (Et3N) as a catalyst. Et3N is a nucleophile and helps to reorganize the cross-linker structure to obtain free ester and carboxyl groups that can react with the hydroxyl groups of CDs [62][63][64]. Since CDI might react with only one CD, fresh CDI-based nanosponges still contain slight amounts of active imidazolyl carbonyl groups, resulting in the formation of dead ends in the polymer structure. This can be avoided by treating CDNSs with water, which enables an almost full elimination of imidazolyl carbonyl groups in 8 h [65]. A similar situation occurs for DPC and phenol moieties as residuals of the synthesis process.
Every method of synthesis includes a suitable purification process, where the various unreacted substrates, byproducts, solvents, and impurities are removed [27]. The most frequently used purification method is Soxhlet extraction using water or organic solvents (ethanol or acetone) [14]. Water removes unreacted CDs, while the ethanol/acetone extraction eliminates the unreacted cross-linker and impurities [18][65].
The four commonly used synthesis approaches have easily modifiable parameters, creating transparent differences between synthesis runs. More sophisticated methods are also in use and worth mentioning; however, they are often related to more complex chemistry or require the application of specialized equipment. The emulsion solvent diffusion method relies on the emulsification process, where the internal phase (consisting of a mixture of drug and CD in a volatile solvent) is added dropwise to the external phase (emulsifying solution) with continuous stirring until evaporation of the internal-phase solvent [60][66]. In the interfacial condensation method, the nanosponges are formed at the interface of a strongly alkaline phase (usually potassium hydroxide solution with pH > 10) with dissolved CD and an organic phase containing the cross-linking agent [67][68]. A more environmentally friendly approach is provided by mechanochemical synthesis, where no solvent is used. Instead, the synthesis is driven by the application of mechanical forces, which provide the energy necessary for the formation of chemical bonds. These types of syntheses are carried out by ball-milling (on a small scale) or using a twin-screw extruder (on a large scale) [65][69].
The synthesis of CDNSs requires the use of appropriate amounts of CD and the cross-linker. The precise mass of each substrate is not significant, but their molar ratio gives valid information about chemical composition.
The structure of synthesized CDNSs can vary at the molecular level, due to different 1:n values. The appropriate synthesis method and conditions are key factors to obtain more rigid structures, related to the higher degree of probe crystallinity. The creation of conditions appropriate for an efficient polymerization process results from even homogenization of the mixture. Thus, using methods with high-intensity mixing, e.g., ultrasound- or microwave-assisted methods, one could produce nanosponges with a highly organized and rigid crystalline structure, whereas basic methods like the melting method do not provide a suitable degree of homogenization, resulting in the formation of CDNSs with a geometrically less-organized, paracrystalline structure [70].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25063527
References
- EudraVigilance eXtended Medicinal Product Dictionary (XEVMPD)—Pharmaceutical Dose Forms. Available online: https://www.ema.europa.eu/en/data-medicines-iso-idmp-standards-post-authorisation/reporting-requirements-marketing-authorisation-holders/guidance-documents-related-data-submission-authorised-medicines (accessed on 24 November 2023).
- Drug Formulation Market by Dosage Form, Indication, End User & Region|Forecast 2022 to 2032. Available online: https://www.futuremarketinsights.com/reports/drug-formulation-market (accessed on 24 November 2023).
- Oral Solid Dosage Pharmaceutical Formulation Market by Dosage Form, Drug Release Mechanism, Distribution Channel & Region|Forecast 2022 to 2032. Available online: https://www.futuremarketinsights.com/reports/oral-solid-dosage-pharmaceutical-formulation-market (accessed on 24 November 2023).
- Swaminathan, S.; Trotta, F. Cyclodextrin Nanosponges. In Nanosponges; Wiley: Hoboken, NJ, USA, 2019; pp. 27–57.
- Gulati, S.; Nigam, A.; Kumar, S. Different Types of Nanosponges Used in Environmental Remediation. In Nanosponges for Environmental Remediation; Gulati, S., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 31–47.
- Atchaya, J.; Girigoswami, A.; Girigoswami, K. Versatile Applications of Nanosponges in Biomedical Field: A Glimpse on SARS-CoV-2 Management. Bionanoscience 2022, 12, 1018–1031.
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537.
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Nanocarrier mediated combination drug delivery for chemotherapy—A review. J. Drug Deliv. Sci. Technol. 2017, 39, 362–371.
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025.
- Rajput, K.N.; Patel, K.C.; Trivedi, U.B. β-Cyclodextrin Production by Cyclodextrin Glucanotransferase from an Alkaliphile Microbacterium terrae KNR 9 Using Different Starch Substrates. Biotechnol. Res. Int. 2016, 2016, 2034359.
- Osmani, R.A.; Kulkarni, P.; Manjunatha, S.; Gowda, V.; Hani, U.; Vaghela, R.; Bhosale, R. Cyclodextrin Nanosponges in Drug Delivery and Nanotherapeutics. In Environmental Nanotechnology; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World Volume 14; Springer: Cham, Switzerland, 2018; Volume 1, pp. 279–342.
- Deng, J.; Chen, Q.J.; Li, W.; Zuberi, Z.; Feng, J.X.; Lin, Q.L.; Ren, J.L.; Luo, F.J.; Ding, Q.M.; Zeng, X.X.; et al. Toward improvements for carrying capacity of the cyclodextrin-based nanosponges: Recent progress from a material and drug delivery. J. Mater. Sci. 2021, 56, 5995–6015.
- Vyas, A.; Saraf, S.; Saraf, S. Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42.
- Chilajwar, S.V.; Pednekar, P.P.; Jadhav, K.R.; Gupta, G.J.C.; Kadam, V.J. Cyclodextrin-based nanosponges: A propitious platform for enhancing drug delivery. Expert Opin. Drug. Deliv. 2014, 11, 111–120.
- Loftsson, T.; Olafsdottir, B.J.; Frioriksdottir, H.; Jonsdottir, S. Cyclodextrin complexation of NSAIDs—Psychicochemical characteristics. Eur. J. Pharm. Sci. 1993, 1, 95–101.
- Ma, M.; Li, D.Q. New organic nanoporous polymers and their inclusion complexes. Chem. Mater. 1999, 11, 872–874.
- Fromming, K.H.; Szejtli, J. Cyclodextrins in Pharmacy; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 1–18.
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406.
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845.
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49.
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180.
- Felton, L.A.; Popescu, C.; Wiley, C.; Esposito, E.X.; Lefevre, P.; Hopfinger, A.J. Experimental and Computational Studies of Physicochemical Properties Influence NSAID-Cyclodextrin Complexation. AAPS PharmSciTech 2014, 15, 872–881.
- Nishikawa, S.; Kondo, M.; Kamimura, E.; Xing, S.Y. Ultrasonic relaxation associated with inclusion complex of drugs and beta-cyclodextrin. Bull. Chem. Soc. Jpn. 2007, 80, 694–698.
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046.
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362.
- Ai, F.; Wang, J.; Li, Y.; Ma, Y. Effect of Drug Particle Size on Complexation, Physicochemical Properties and Dissolution of Cyclodextrin Inclusion Complexes. Indian J. Pharm. Sci. 2017, 79, 131–138.
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479.
- Ahmed, R.Z.; Patil, G.; Zaheer, Z. Nanosponges—A completely new nano-horizon: Pharmaceutical applications and recent advances. Drug Dev. Ind. Pharm. 2013, 39, 1263–1272.
- Li, D.; Ma, M. Nanoporous polymers: New nanosponge absorbent media. Filtr. Sep. 1999, 36, 26–28.
- Szejtli, J. The properties and potential uses of cyclodextrin derivatives. J. Inclusion Phenom. Mol. Recognit. Chem. 1992, 14, 25–36.
- Pawar, S.; Shende, P.; Trotta, F. Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350.
- Allahyari, S.; Trotta, F.; Valizadeh, H.; Jelvehgari, M.; Zakeri-Milani, P. Cyclodextrin-based nanosponges as promising carriers for active agents. Expert Opin. Drug. Deliv. 2019, 16, 467–479.
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621.
- Adeoye, O.; Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: A mini review. Int. J. Pharm. 2017, 531, 521–531.
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-based nanosponges for drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213.
- Rossi, B.; Caponi, S.; Castiglione, F.; Corezzi, S.; Fontana, A.; Giarola, M.; Mariotto, G.; Mele, A.; Petrillo, C.; Trotta, F.; et al. Networking Properties of Cyclodextrin-Based Cross-Linked Polymers Probed by Inelastic Light-Scattering Experiments. J. Phys. Chem. B 2012, 116, 5323–5327.
- Pawar, S.; Shende, P. A Comprehensive Patent Review on beta-cyclodextrin Cross-linked Nanosponges for Multiple Applications. Recent Pat. Nanotechnol. 2020, 14, 75–89.
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099.
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaccutical solubilizers. Adv. Drug Del. Rev. 2007, 59, 645–666.
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug. Deliv. 2005, 2, 335–351.
- Tiwari, K.; Bhattacharya, S. The ascension of nanosponges as a drug delivery carrier: Preparation, characterization, and applications. J. Mater. Sci. Mater. Med. 2022, 33, 28.
- Feng, J.F.; Tan, M.; Zhang, S.; Li, B.J. Recent Advances of Porous Materials Based on Cyclodextrin. Macromol. Rapid Commun. 2021, 42, 2100497.
- Pawar, A.Y.; Naik, A.K.; Jadhav, K.R. Nanosponges: A Novel Drug Delivery System. Asian J. Pharm. 2016, 10, 456–463.
- Flourie, B.; Molis, C.; Achour, L.; Dupas, H.; Hatat, C.; Rambaud, J.C. Fate of beta-cyclodextrin in the human intestine. J. Nutr. 1993, 123, 676–680.
- Antenucci, R.N.; Palmer, J.K. Enzymatic degradation of alpha-cyclodextrins and beta-cyclodextrins by Bacteroides of the human colon. J. Agric. Food. Chem. 1984, 32, 1316–1321.
- Osmani, R.A.M.; Bhosale, R.R.; Hani, U.; Vaghela, R.; Kulkami, P.K. Cyciodextrin Based Nanosponges: Impending Carters in Drug Delivery and Nanotherapeutics. Curr. Drug Ther. 2015, 10, 3–19.
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech. Chem. Eng. 2023, 67, 11–17.
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble beta-cyclodextrin-epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368.
- Krabicova, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122.
- Appleton, S.L.; Tannous, M.; Argenziano, M.; Muntoni, E.; Rosa, A.C.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as protein delivery systems: Insulin, a case study. Int. J. Pharm. 2020, 590, 119888.
- Deshmukh, K.; Tanwar, Y.S.; Sharma, S.; Shende, P.; Cavalli, R. Functionalized nanosponges for controlled antibacterial and antihypocalcemic actions. Biomed. Pharmacother. 2016, 84, 485–494.
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361.
- Deshmukh, K.; Tanwar, Y.S.; Shende, P.; Cavalli, R. Biomimetic estimation of glucose using non-molecular and molecular imprinted polymer nanosponges. Int. J. Pharm. 2015, 494, 244–248.
- Nazerdeylami, S.; Ghasemi, J.B.; Ziarani, G.M.; Amiri, A.; Badiei, A. Direct monitoring of diclofenac using a supramolecular fluorescent approach based on beta-cyclodextrin nanosponge. J. Mol. Liq. 2021, 336, 116104.
- Cavalli, R.; Akhter, A.K.; Bisazza, A.; Giustetto, P.; Trotta, F.; Vavia, P. Nanosponge formulations as oxygen delivery systems. Int. J. Pharm. 2010, 402, 254–257.
- Trotta, F.; Cavalli, R.; Martina, K.; Biasizzo, M.; Vitillo, J.G.; Bordiga, S.; Vavia, P.; Ansari, K. Cyclodextrin nanosponges as effective gas carriers. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 189–194.
- Femmino, S.; Penna, C.; Bessone, F.; Caldera, F.; Dhakar, N.; Cau, D.; Pagliaro, P.; Cavalli, R.; Trotta, F. alpha-Cyclodextrin and alpha-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers 2018, 10, 211.
- Peila, R.; Scordino, P.; Shanko, D.B.; Caldera, F.; Trotta, F.; Ferri, A. Synthesis and characterization of beta-cyclodextrin nanosponges for N,N-diethyl-meta-toluamide complexation and their application on polyester fabrics. React. Funct. Polym. 2017, 119, 87–94.
- Mihailiasa, M.; Caldera, F.; Li, J.M.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded beta-cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30.
- Jain, A.; Prajapati, S.K.; Kumari, A.; Mody, N.; Bajpai, M. Engineered nanosponges as versatile biodegradable carriers: An insight. J. Drug Deliv. Sci. Technol. 2020, 57, 101643.
- Anandam, S.; Selvamuthukumar, S. Optimization of microwave-assisted synthesis of cyclodextrin nanosponges using response surface methodology. J. Porous Mater. 2014, 21, 1015–1023.
- Singh, P.; Ren, X.H.; Guo, T.; Wu, L.; Shakya, S.; He, Y.P.; Wang, C.F.; Maharjan, A.; Singh, V.; Zhang, J.W. Biofunctionalization of beta-cyclodextrin nanosponges using cholesterol. Carbohydr. Polym. 2018, 190, 23–30.
- Singh, V.; Xu, J.H.; Wu, L.; Liu, B.T.; Guo, T.; Guo, Z.; York, P.; Gref, R.; Zhang, J.W. Ordered and disordered cyclodextrin nanosponges with diverse physicochemical properties. RSC Adv. 2017, 7, 23759–23764.
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged beta-Cyclodextrin Nanosponges. Materials 2021, 14, 478.
- Pedrazzo, A.R.; Caldera, F.; Zanetti, M.; Appleton, S.L.; Dahkar, N.K.; Trotta, F. Mechanochemical green synthesis of hyper-crosslinked cyclodextrin polymers. Beilstein J. Org. Chem. 2020, 16, 1554–1563.
- Gangadharappa, H.V.; Prasad, S.M.C.; Singh, R.P. Formulation, in vitro and in vivo evaluation of celecoxib nanosponge hydrogels for topical application. J. Drug Deliv. Sci. Technol. 2017, 41, 488–501.
- Shende, P.; Kulkarni, Y.A.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and Repeated Dose Toxicity Studies of Different beta-Cyclodextrin-Based Nanosponge Formulations. J. Pharm. Sci. 2015, 104, 1856–1863.
- Desai, D.; Shende, P. Drug-Free Cyclodextrin-Based Nanosponges for Antimicrobial Activity. J. Pharm. Innov. 2021, 16, 258–268.
- Pedrazzo, A.R.; Trotta, F.; Hoti, G.; Cesano, F.; Zanetti, M. Sustainable mechanochemical synthesis of beta-clodextrin polymers by twin screw extrusion. Environ. Sci. Pollut. Res. Int. 2022, 29, 251–263.
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201.
This entry is offline, you can click here to edit this entry!
