Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Endocrinology & Metabolism
Heart failure (HF) is a chronic disorder of the cardiovascular (CV) system and remains a major cause of morbidity and mortality worldwide. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors are beneficial for patients suffering from type 2 diabetes mellitus (T2DM) or established cardiovascular disease (CVD), mainly HF, by reducing CVD-related morbidity and mortality.
- SGLT-2 inhibitors
- acute decompensated heart failure
- diuretics
1. Diuretic Resistance in HF
HF exacerbation is mainly associated with symptoms from excess extracellular fluid volume. The development of acute decompensated HF leads to poor outcomes and an impaired quality of life. Even now, diuretics are the cornerstone of acute decompensated HF treatment and the most commonly described symptomatic drug therapy for chronic congestive HF when decongestion is necessary [18,19]. Patients with HF are frequently hospitalized and more than 50% exit the hospital without sufficient weight loss and with residual congestion. Although there is not a universally accepted definition, the failure to achieve appropriate congestion relief with low urine sodium concentration, despite using adequate or escalating diuretic doses, is described as diuretic resistance (DR) [7,10]. DR is considered a well-established factor of worsening HF, the prolongation of hospital stays, higher readmission rates, and increased morbidity and mortality [7,10].
In patients with acute decompensated or chronic congestive HF, DR derives from a variety of mechanisms [20] (Figure 1). One well-established mechanism is the compensatory post-diuretic sodium reabsorption (CPDSR) and post-diuretic retention [18]. High doses of loop diuretics induce the remodeling of the distal nephron, including hypertrophy and hyperplasia of the distal convoluted tubule, the connecting tubule, and the collecting ducts. When administering loop diuretics for long periods, sodium absorption is obstructed in the loop of Henle, but may be enhanced in the hypertrophied distal tubule, resulting in an unchanged sodium balance [18]. Moreover, the initial fluid loss is followed by the activation of the renin–angiotensin–aldosterone system (RAAS) and of the sympathetic nervous system (SNS), resulting in post-diuretic sodium retention and mitigating the beneficial outcomes of loop diuretics [18,20].
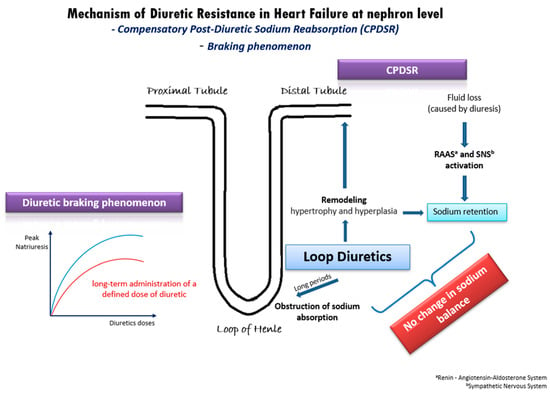
Figure 1. Underlying mechanisms of diuretic resistance in heart failure.
Another possible pathophysiologic mechanism of DR in HF is the diuretic braking phenomenon [21]. This phenomenon arises from the long-term administration of a specific dose of diuretic medication, leading to a gradual decline in peak natriuresis over time. This decline can be partially attributed to structural changes (remodeling) in the nephrons induced by the prolonged use of high doses of loop diuretics. Specifically, the dose–response curve in congestive HF shifts downwards and to the right (Figure 1), indicating a reduction in the maximum achievable natriuresis and an increase in the dose of diuretics required to achieve similar diuretic effects [21]. As in post-diuretic retention, the braking phenomenon is inextricably intertwined with neurohormonal activation and RAAS activation. Additionally, it is well-established that loop diuretics can initiate renin secretion through the obstruction of the sodium–potassium–chloride cotransporter, by enhancing prostacyclin production and by inducing volume contraction [22]. Overall, the diuretic braking phenomenon represents a complex interplay of structural and biochemical changes within the kidney, neurohormonal activation, and RAAS activity, contributing to the development of DR in patients with HF.
2. The Intersection of SGLT-2 Inhibitors and Diuretic Resistance
2.1. Pathophysiology and Mechanisms
The diuretic effect of SGLT-2 inhibitors occurs in the proximal convoluted tubule (PCT), where SGLT2s are located. SGLT-2 inhibitors reduce sodium reabsorption by causing the constriction of the proximal arteriole and the dilatation of the distal arteriole, lowering the GFR. SGLT-2 inhibitors also reduce proteinuria and hyperfiltration [20]. Because the majority of sodium is reabsorbed in the loop of Henle and the distal tubule, SGLT-2 inhibitors have minimal diuretic effects, but they can enhance the diuretic response when combined with other classes of diuretics by improving the responsiveness to atrial natriuretic peptide [20].
Preliminary clinical data from trials using SGLT-2 inhibitors in patients with acute HF needing diuresis are encouraging. In a double-blind, randomized, controlled trial, empagliflozin increased urinary output only for four days in ADHF patients, but it reduced mortality, HF rehospitalization, and in-hospital-worsening HF at 60 days [37]. In a small retrospective study recruiting 31 patients with T2DM who received SGLT-2 inhibitors as adjuvant therapy, weight loss, urine volume, and diuretic efficiency was improved 24 h after initiation, without the worsening of renal function, potassium, or blood pressure [38]. The diuretic synergy of dapagliflozin and bumetanide was evaluated in healthy subjects, in whom the diuretic effect and Na+ excretion was enhanced when one drug treatment was added on the other after a week of single-drug treatment [39].
Τhe underlying mechanisms through which SGLT-2 inhibitors may mitigate diuretic resistance are multifactorial and involve intricate physiological processes (Figure 2). These pharmacological agents exert their effects through a cascade of interconnected pathways, each contributing to the overall therapeutic outcome. Their effects on natriuresis, renal function, SNS activation, and hemodynamics play a pivotal role [40]. Firstly, SGLT-2 inhibitors exhibit a pronounced impact on natriuresis, the excretion of sodium in the urine, thereby promoting the elimination of excess fluid from the body. By inhibiting the reabsorption of glucose and sodium in the proximal renal tubules, these agents enhance the urinary excretion of both substances, leading to a net reduction in extracellular fluid volume. Moreover, the renal effects of SGLT-2 inhibitors extend beyond simple natriuresis. They modulate renal function by influencing the GFR and tubuloglomerular feedback mechanisms. By enhancing the GFR and altering tubular dynamics, these agents contribute to the overall regulation of fluid balance and renal hemodynamics. Animal studies have also presented evidence of the suppression of the SNS by moderating the adrenergic activity of the afferent sympathetic nerve, resulting in the reduced activation of the RAAS [40]. SGLT-2 inhibitors have an additional distinctive attribute to shrink the interstitial fluid volume greater than the intravascular fluid volume. This mechanism acts protectively against the neurohormonal activation induced by alterations in intravascular fluid volume [40].
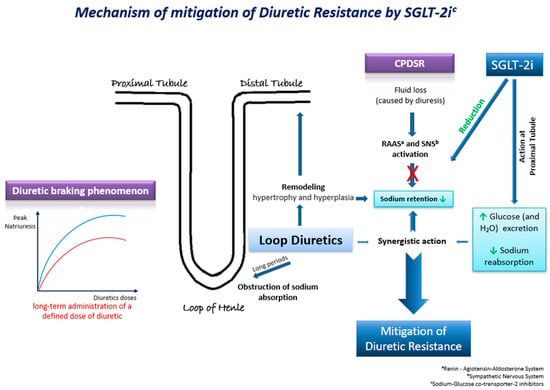
Figure 2. Τhe possible underlying mechanisms through which SGLT-2 inhibitors may mitigate diuretic resistance in HF.
Furthermore, SGLT-2 inhibitors have shown undeniably beneficial effects on the kidney. In an experimental study involving rats, dapagliflozin exhibited a remarkable reduction in inflammatory processes and fibrotic changes within the nephron, indicating a potential mechanism mediated by the activation of sirtuin-1 (SIRT1) and subsequent inhibition of nuclear factor-κB (NF-κB) expression. This intricate pathway not only mitigates immediate renal damage but also holds promise for attenuating the progression of chronic kidney disease by curbing long-term oxidative stress [41].
Moreover, SGLT-2 inhibition offers additional advantages through its effects on erythropoiesis and hematocrit levels. It has been proposed that SGLT-2 inhibitors modulate medullary oxygen tension, which in turn influences the function of myofibroblasts within the renal interstitium. This alteration in myofibroblast function promotes the synthesis and release of erythropoietin, leading to an elevation in erythropoietin levels and subsequent increase in hematocrit. The rise in hematocrit not only enhances oxygen-carrying capacity systemically, but also plays a crucial role in improving oxygen delivery specifically to the renal cortex and medulla. This enhancement in renal tissue oxygenation is vital for maintaining optimal renal function and may contribute to the preservation of renal structure and function over the long term [41].
2.2. Efficacy Data
Several clinical studies have investigated the impact of SGLT-2 inhibitors on the attenuation of diuretic resistance in patients with HF (Table 1). SGLT-2 inhibitors seem to have a moderate diuretic effect (increases in urine output), a result that has been observed repeatedly when they are integrated in the conventional treatment of HF patients with loop diuretics [42,43,44]. This diuretic effect is mainly attributed to the combination of induced glycosuria causing osmotic diuresis and the resultant activation of compensatory mechanisms, which in turn halts the diuretic effect of SGLT-2 inhibitors [39,44] and occurs mostly at the early stages of treatment. This effect diminishes around the 24 h mark after the initiation of SGLT-2 inhibitors and is not sustained in the long term [38,39,42,43,44,45,46,47]. These findings are promising for the use of SGLT-2 inhibitors as an effective add-on therapy to standard diuretic medication among patients hospitalized with ADHF.
Moreover, the diuresis itself seems to not be linked with natriuresis, as most short-term studies show no significant alterations in urinary sodium excretion and the evidence does not support a sustained natriuretic effect of SGLT-2 inhibitors [38,39,42,43,44,45,46,47]. There is only one study of 20 euvolemic HF patients with DR, which documented a more sustained natriuretic effect of empagliflozin that remained for up to 2 weeks, which led to a significant decrease in blood volume [39]. Interestingly, some studies report that when empagliflozin was used in combination with bumetanide, there was a compound effect in respect to fractional sodium excretion in urine, suggesting a synergistic effect of these drugs to natriuresis [39]. These results are in agreement with the mechanism of action of SGLT-2 inhibitors, which presents them more as modulators of volume that help the nephron manage sodium and fluid more efficiently, rather than as pure diuretic agents [48].
Apart from their impact on fluid balance, SGLT-2 inhibitors lead to significant decreases in body weight (BW) when included in conventional therapy, compared to a placebo [39,42,49]. However, it is not yet clear that the observable decreases in BW are completely due to decongestion [44]. Some trials suggest that the decline in BW could be a result of a loss of calories in urine, because of the glycosuria caused by SGLT-2 inhibitors [38,44]. For instance, according to findings from the EMPA-RESPONSE-AHF trial, despite the elevation in urinary output, empagliflozin was not associated with decongestion signs (either with improved symptoms of dyspnea (p = 0.18) or improved diuretic response (−0.35 ± 0.44 vs. −0.12 ± 1.52 kg/40 mg furosemide equivalents; p = 0.37)) over the first 4 days compared to the control group [38].
Concomitantly, with their effects on fluid balance, SGLT-2 inhibitors elicit elevations in hematocrit levels among HF patients when incorporated into conventional therapeutic regimens, as evidenced by multiple trials [44,46,48]. These investigations indicate that the mechanism underlying this hematocrit increase predominantly stems from heightened erythropoietin production and subsequent erythropoiesis, rather than solely from hemoconcentration induced by diuresis and volume depletion [44,46,50,51].
In addition, SGLT-2 inhibitors improve the symptomatology of HF patients, when assessed using the KCCQ-TSS or other congestion scores [52,53]. These results were documented mostly 12 weeks after the beginning of treatment [44,53,54]. Interestingly, the results stemming from the EMPULSE trial suggest that the improvement in symptomatology in patients across the KCCQ-TSS can be documented as early as 15 or 30 days after the initiation of SGLT-2 inhibitors [52]. However, none of the aforementioned studies reported improvement in respect to objective markers of congestion such as jugular venous distension, edema, ascites, or pleural effusion [44], while those that did showed no significant changes [44,54,55]. The lack of objective or patient-reported markers of decongestion was further highlighted by the fact that no stable correlation was observed when examining the levels of natriuretic peptides (in particular NT-proBNP) alongside the improvement in clinical briefing when administrating an SGLT-2 inhibitor. Some studies reported no change regarding NT-proBNP levels [43,44,50,54,55], and on the other hand, a few studies reported a reduction in NT-proBNP [43,44,50,52]. The heterogeneity of the results in the given trials indicates that there is no solid evidence to support the theory that the beneficial effects of these drugs in the symptomatology of HF patients, or even in the reduction in the risk of death or hospitalization, are solely due to a distinct action in renal function and successful decongestion. This suggests that there may be a different underlying mechanism of action [44,56].
Other important findings referred to the dosage of loop diuretics, which generally required less intensification with the co-administration of SGLT-2 inhibitors compared to the control group [50,52,57]; however, at the same time, their impact on the frequency of the de-escalation of the baseline dosage or of the loop diuretic discontinuation between treatment arms was ambiguous [39,49,58]. Moreover, in one study, the possibility of adding another loop diuretic dropped significantly with the addition of an SGLT-2 inhibitor in the treatment of HF patients, and those results, along with other clinical benefits and the observed decreases in diuretics dosage, were independent of the type or the dosage of the diuretics used in the trial [58]. One possible underlying mechanism is the synergistic diuretic effect of SGLT-2 inhibitors when added on loop diuretics. Indeed, according to findings from a single-center, double-blind, placebo-controlled RCT (EMPAG-HF), the efficacy of furosemide was significantly increased in the SGLT-2 inhibitor arm (14.1 mL urine/mg furosemide equivalent, 95% CI: [0.6–27.7]; p = 0.041) [43]. However, when compared to other diuretics (e.g., metolazone), SGLT-2 inhibitors were less effective (non-significance) at relieving congestion when added to intravenous loop diuretics in patients with HF and DR (MD −0.08 kg, 95% CI: [−0.17–0.01]; p = 0.10) [47].
Table 1. Key studies investigating the impact of SGLT-2 inhibitors on the attenuation of diuretic resistance in patients with HF.
| Study ID | Type of Study | Population (Main Characteristics) | SGLT2 Inhibitor vs. Comparator | Follow-Up | Main Outcomes |
|---|---|---|---|---|---|
| Yeoh et al., 2023 [47] | Multicenter, open-label, randomized, parallel group trial | 61 patients hospitalized for HF with resistance to treatment with iv loop diuretics (furosemide) | Dapagliflozin 10 mg/day vs. metolazone 5–10 mg once daily. Randomization 1:1. Duration of treatment 3 days. | 5 days |
|
| Biegus et al., 2023 [52] | Prespecified secondary analysis of the multicenter, double-blind RCT (EMPULSE trial) |
530 patients hospitalized due to symptoms and signs of ADHF requiring iv loop diuretics after initial stabilization | Empagliflozin 10 mg/day vs. placebo as add-on therapy for 3 months. Randomization 1:1. |
90 days |
|
| Chatur et al., 2023 [58] | Prespecified subgroup analysis of the DELIVER multicenter RCT | 6263 patients with HFpEF and at least intermittent diuretic requirement, divided in three groups: (i) No-diuretic (10.9%), (ii) Non-loop diuretic (12.3%), (iii) Loop-diuretic (76.8%) (furosemide < 40 mg, 40 mg and >40 mg) | Dapagliflozin 10 mg/day vs. placebo. Randomization 1:1 |
3 years |
|
| Charaya et al., 2022 [49] | Single-center, open-label, randomized pilot study | 102 patients hospitalized for ADHF and requiring iv administration of loop diuretics, with LVEF between 30.2% and 59.6% and eGFR 32.1–71.1 mL/min | Dapagliflozin 10 mg/day in addition to standard diuretics vs. conventional therapy. Randomization 1:1 | 6 days intrahospital and 30 days after discharge |
|
| Mordi et al., 2020 [42] | Single-center, double-blind, placebo-controlled, crossover RCT (RECEDE-CHF trial) | 23 patients with T2DM and HFrEF and furosemide dose of 18.3–80.9 mg/day | Empagliflozin 25 mg/day vs. placebo as add-on therapy to regular loop diuretic for 6 weeks. Then, 2 weeks as a washout period, and then drug switch between the two groups and treatment for 6 more weeks. Randomization 1:1. |
6 + 6 weeks |
|
| Schulze et al., 2022 [43] | Single-center, double-blind, placebo-controlled, RCT (EMPAG-HF) | 60 patients hospitalized for ADHF and requiring loop diuretics administration | Empagliflozin 25 mg/day vs. placebo as add-on therapy to regular loop diuretic. Randomization 1:1. |
5 days (efficacy) and 30 days (safety outcomes) |
|
| Damman et al., 2020 [39] | Multicenter, double-blind, placebo-controlled pilot study (EMPA-RESPONSE-AHF) | 79 patients hospitalized for acute HF requiring loop diuretics administration | Empagliflozin 10 mg/day vs. placebo for 1 month. Randomization 1:1. |
30 days |
|
| Tamaki et al., 2021 [47] | Single-center, open-label RCT | 59 patients with T2DM hospitalized for AHF requiring loop diuretics administration | Empagliflozin 10 mg/day vs. standard antidiabetic treatment. Randomization 1:1. |
1 week |
|
| Packer et al., 2021 a [57] | Multicenter, double-blind, RCT (EMPEROR-Preserved trial) | 5988 patients with HF and EF > 40% | Empagliflozin 10 mg/day vs. placebo as an add-on to conventional therapy. Randomization 1:1. |
26 months |
|
| Packer et al., 2021 b [50] | Multicenter, double-blind, RCT (EMPEROR-Reduced trial) | 3730 patients with HFrEF (39.6% in “volume overload” and 57% “euvolemic”). | Empagliflozin 10 mg/day vs. placebo as an add-on to conventional therapy. Randomization 1:1. |
720 days |
|
| Griffin et al., 2020 [59] | Case series (retrospective analysis) | 31 patients with ADHF (58% HFrEF) and DR despite loop diuretics administration | SGLT2 inhibitor (canagliflozin or empagliflozin) vs. control as an add-on diuretic therapy | 3 days |
|
2.3. Safety Profile and Adverse Effects
The occurrence of common side effects was usually balanced between SGLT-2 inhibitors and placebo comparator treatment arms, and serious adverse effects that led to the discontinuation of the treatment were scarce, suggesting that these drugs are generally well tolerated [42,43,47,50,57,59]. The most common side effects reported were increased urination, genital mycotic infections, urinary tract infections, and volume depletion phenomena [37,43,49,53,54,58,59]. Similar findings have been previously attributed to SGLT-2 inhibitors due to glycosuria [60]. Mild volume depletion (presented with symptomatic hypotension or orthostatic hypotension, polyuria, dehydration, dizziness, vertigo, presyncope, thirst, and rarely orthostatic hypotension), weight loss, a reduction in SBP, the potential deterioration of renal function (increases in serum creatinine levels, decreases in the eGFR), acute kidney injury or failure, potential changes in hematocrit and hemoglobulin, liver function deterioration, diabetic ketoacidosis, and hypoglycemia can also be attributed to the pharmacology of the SGLT-2 inhibitor class [37,50,57,61], which causes osmotic diuresis, natriuresis, glucosuria, and caloric wasting [60]. For instance, in their study, Wilcox et al. reported two cases, one with syncope attributed possibly to orthostatic hypotension and another presented with hypokalemia that required oral potassium chloride to be administered [39]. It is worth noting that in the same study, most of the adverse effects were mild and similar between the patients receiving dapagliflozin and those receiving a placebo [39].
2.3.1. Deterioration of Kidney Function
SGLT-2 inhibitors are considered relatively safe drugs that could actually provide a reno-protective effect rather than harming the kidneys [37,43,49,53,54,57,59,61]. Indeed, although there are a handful of studies that refer to small decreases in the eGFR, which have been observed shortly after the addition of an SGLT-2 inhibitor in the medication of patients hospitalized for ADHF [45,49], the deterioration of renal function was mostly not persistent after hospital discharge [45,49,62]. A trial with a total of 23 patients reported two cases with an acute increase in serum creatinine, which was attenuated later at six weeks [42]. On the other hand, in their study, Schulze et al. demonstrated that markers of renal injury (urinary protein, creatinine, and urinary a1-microglobulin) were similar in both the SGLT-2 inhibitor and the placebo group, suggesting no significant deterioration of the kidneys [43]. In addition, the same study reported no difference between the mean value of the eGFR between patients receiving the SGLT-2 inhibitor and those receiving the placebo [43]. Another study documented a slower rate of eGFR decline in patients receiving empagliflozin [57].
2.3.2. Co-Administration with Other Diuretics
When SGLT-2 inhibitors are given as a standalone treatment, there is a compensatory increase in sodium reabsorption in the distal tubules, which are the target site of thiazides. This occurs after the loop of Henle, and as a result, the actual diuretic effect is constrained. However, the use of SGLT-2 inhibitors in combination with thiazides can lead to substantial diuretic effects [63]. The compensatory reabsorption of sodium in the distal tubules relies on the presence of aldosterone [64]. As a result, when SGLT-2 inhibitors are administered alongside an aldosterone antagonist, it not only inhibits compensatory reabsorption in the distal tubules, but also interferes with reabsorption in the collecting duct (the site where aldosterone antagonists act), resulting in even more enhanced diuresis [65]. Particular attention should be directed towards the findings of the EMPA-REG OUTCOME trial sub-analysis [66], which indicate that the combination of SGLT-2 inhibitors and aldosterone antagonists may worsen HF, potentially due to dehydration and reduced cardiac output [65].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25063122
This entry is offline, you can click here to edit this entry!
