Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Paraoxonase enzymes have a protective role due to their ability to contribute to antioxidant and anti-inflammatory pathways, especially paraoxonase 1 (PON1). PON1 is diminished in infectious diseases, it can be used as a marker, and it can lead to lower protection against the microorganism; therefore, it can play a part in the development of antibiotic resistance.
- paraoxonase 1 (PON1)
- endothelial dysfunction
- oxidative stress
- dyslipidemia
1. Endothelial Dysfunction
Endothelial dysfunction describes a state when the normal proprieties of the endothelium are disturbed, leading to a pro-inflammatory and prothrombotic state, abnormal vasodilatation, leukocyte adhesion, and higher permeability [1][2]. Nitric oxide is a soluble gas with a major role in vascular homeostasis, being a strong vasodilatory, anti-inflammatory, and antioxidant substance [3]. Free radicals can damage the balance of NO concentration and the endothelial barrier, allowing other substances to enter the body [1]. Increased levels of reactive oxygen species (ROS) are responsible for cell damage and death resulting from an imbalance in pro- and antioxidative species in favor of pro-oxidant ones. Oxidative stress is behind many diseases due to its major role in inflammation and fibrogenesis [4]. Endothelial dysfunction drives peripheral vascular disease through abnormal NO levels, which means an increased state of vasoconstriction, leading to ischemia and therefore a discordant oxygen delivery demand. Plaque rupture also results from endothelial dysfunction, generating critical limb ischemia [1]. NO levels are maintained in normal parameters, but when dysfunction occurs, lower or higher levels lead to different outcomes; for example, during myocardial infarction, high NO levels have negative inotropic effects [3].
Platelets are a key member of atherosclerosis, being the main cells in the first stage of thrombus formation. The adhesion, activation, and aggregation of platelets do not occur if the endothelium is intact, only when it has a breach or in other specific situations. When the endothelium is exposed, it interacts with the endothelium, collagen, thromboxane, and ADP (adenosine diphosphate), and thrombin activates them. As a result, they secrete chemotaxins, coagulation factors, and vasoconstrictors, inducing more platelet activation [1]. Although for a long time it was believed that platelets only adhere to the endothelium if there is damage, the reality is that they can stick even if it is inflamed or if it is a location that is prone to adhesion such as the carotid bifurcation [1].
Hypertension is also combined with endothelial dysfunction, but it remains unknown if it is the cause or result. At first, it was believed that endothelial dysfunction occurs because of the chronic increased pressure, but studies on hypertensive drugs showed no changes in endothelial dysfunction [1]. A newer study suggests that the effect depends on the antihypertensive classes, because using angiotensin receptor blockers or angiotensin-converting enzyme inhibitors does fight against the dysfunction [5]. High arterial pressure leads to an overproduction or a more ample effect of vasoconstriction agents in contrast to vasodilative factors [1]. NO (nitric oxide) metabolism is also dysbalanced through oxidative stress mediated by angiotensin II. Angiotensin II is responsible for the disturbance between oxidants/antioxidants by increasing the permeability of the vascular lumen [2][6].
An association between endothelial dysfunction leading to inflammation and atherosclerosis is given by atherosclerosis-associated endothelial–leukocyte adhesion molecule (VCAM-1). In animal models, it has been found that in coronary arteries, VCAM-1 lays on the intact endothelium, surfacing the plaque, and is usually found in areas prone to lesions. Moreover, VCAM-1’s isoform plasma levels correlate with more serious atherosclerotic lesions [7].
All the factors implicated in atherosclerosis (smoking, hypertension, diabetes, and hypercholesterolemia) are combined with endothelial dysfunction resulting in a pro-inflammatory state, probably due to lower NO levels and therefore increased leukocyte adhesion molecules and cytokines leading to a state prone to arterial lesions.
Firstly, macrophages are internalized into the intima and transformed into lipoprotein and foam cells. Chemokines induce the activity of muscle cells generating a fibromuscular plaque, which will eventually become a fibrous cap with oxidized lipoproteins and inflammatory cells modulating the endothelium and finally leading to an unstable plaque [7]. As Ulf Landmesser and David G. Harrison suggested, lower NO arterial availability is caused by superoxide production, which either degrades NO or the cofactor used in its synthesis [1][8]. Lipids also diminish NO levels because of their power to activate pro-inflammatory pathways like those mediated by NF-kB (nuclear factor kappa-B) [9]. Endothelin plays a role in atherogenesis by the stimulation of pro-inflammatory mediators (IL-6, IL-8, TNF-α (tumor necrosis factor), and superoxide anion), smooth muscle hypertrophy, and increased response to angiotensin II [1][10]. TNF-α stimulates ROS formation, and in coronary arteries, this implies the transformation of stable plaque into unstable plaque, which can lead to a coronary event (Figure 1) [11].
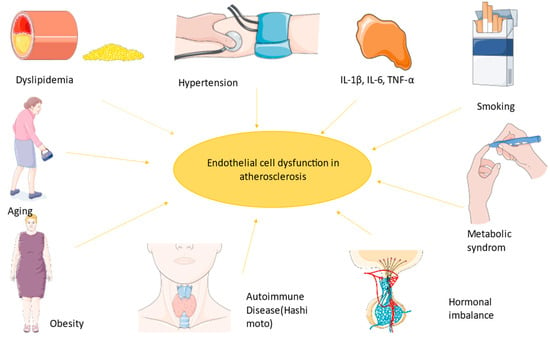
Figure 1. The most important factors implicated in endothelial dysfunction led to a state prone to atherosclerosis. The figure was composed using Servier Medical Art templates and PowerPoint, Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ Accessed on 23 February 2024).
Atherosclerotic lesions can transform into a vulnerable plaque that can break down and lead to thrombosis. Depending on the dimensions of the thrombus and of the vessel, it can lead to stenosis or occlusion, and ultimately end-organ damage [12]. A question that remains is how to assess endothelial dysfunction. The principle is to measure the ability of the cell to stimulate vasodilation or vasoconstriction. This was studied by Sandoo et al. using pulse wave velocity, venous occlusion plethysmography, and flow-mediated vasodilatation in the brachial artery [13][14]. The last one is based on NO (nitric oxide) release, by measuring the diameter of the brachial artery, inducing ischemia with a cuff, and after 5 min, NO causes vasodilatation. This result can be seen when re-measure [11]. Also, the carotid–intima media thickness is used more frequently because it is more accessible, easier to measure, and non-invasive [14]. Newer methods such as epicardial fat thickness, the ankle–brachial index, and arterial stiffness were used for the assessment of endothelial dysfunction [14]. Another way is to measure biomarkers such as IL-1β, IL-6, E-selectin, or VCAM-1-associated CRP (C reactive protein), which are increased in atherosclerotic lesions that can be seen using paraclinical examination such as Doppler echography for peripheral lesions or coronagraphy for coronary atherosclerosis. Other tests used are ischemia-modified albumin (IMA), endothelial cell-specific molecule 1 (Endocan), pentraxin-3 (PTX3), asymmetrical dimethylarginine (ADMA), endothelial microparticles, endothelial progenitor cells (EPCs), angiopoietin-1 (Ang-1), and von Willebrand Factor (vWf) [14]. However, the standard remains FMD (flow-mediated vasodilatation) in response to acetylcholine [2]. The importance of endothelial dysfunction was demonstrated with angiography; during exploration it was seen that a paradoxical vasoconstriction occurred when acetylcholine was administrated in patients with severe atherosclerosis in contrast to the vasodilation in people with normal coronary vessels [7]. This study shows the importance of a deficient response in the circulatory system due to a lack of NO production.
2. PON1 Function
PON1 is a 43 kDa calcium-dependent glycoprotein formed from 355 amino acids and is a member of the PON family with PON2 and PON3, located on chromosome 7, all with a protein structure. It is mainly produced in the liver, as well as in the kidneys, and is usually transported in plasma, being associated with high-density lipoprotein (HDL). Oxidative stress and free radicals have a major role in many pathologies such as neurodegeneration, liver and cardiovascular diseases, diabetes, and most importantly atherogenesis. All of the above are developed by inflammation (TNF-α, IL-1, and IL-6) and oxidative stress. It is well known that antioxidative substances are needed to prevent these pathologies, and it is believed that paraoxonase enzymes have a protective role due to their ability to contribute to antioxidant and anti-inflammatory pathways, especially paraoxonase 1 (PON1) [4][15][16].
PON1 has calcium ions sites; therefore, it needs calcium for activation [15]. If the calcium is removed, then some of the activities dependent on calcium are inactivated, such as POXase and AREase activity, but the oxidative protection of LDL is still ongoing [17]. The three cysteine parts of its structure are used for its function and for creating a disulfide bridge, but most importantly for their role in protecting against LDL oxidation [18]. It is known that PON1 has a big role in stopping the first stage of foam cell formation, and in atherosclerotic plaque geneses by preventing low-density lipoprotein (LDL) oxidation, macrophage formation, and the production of monocyte chemoattractant protein-1 (MCP-1) [15]. Studies on animals showed a difference between the overexpression of PON1 associated with a higher resistance to inflammation and lipid oxidation, whereas those with lower levels were more susceptible to atherosclerosis [4]. Serum levels are different between people because their levels are modified by diet, environment, and diseases [19]. PON3 has also been studied recently, and it has some of the proprieties of PON1, being involved in the prevention of atherosclerosis, but it only has arylesterase and lactonase activity [16][20]. PON2 is more frequently found in females; it also has oxidative properties, and it might be a reason for the protection against a few cardiovascular and neurological diseases [21]. There are some differences in PON1 function and plasma levels depending on the polymorphism of the genes that code PON1. Some changes can be explained by the polymorphism of the amino acids from position 192 (arginine or glutamine), which in this case resulted in the lowest activity of PON1, or there can be some changes in the 55 positions (leucine or methionine). Coronary heart disease is associated with a higher chance of having the R allele or homozygosity of the L allele [18]. Mohamed et al. demonstrated in the Egyptian population with coronary disease that HDL levels were lower and total cholesterol, triglyceride, and LDL cholesterol were higher in those with the PON1 RR allele than those with PON1 QQ, but there was no correlation with the severity of coronary atherosclerosis [22]. Also, those with the G allele of the rs662 single-nucleotide polymorphism have one more added risk factor to develop coronary artery disease, while rs854560 is not linked with it [23][24].
The PON1 natural structure is based on lactone and lipophilic substrates. The aromatic amino acids from the active sites are responsible for the adherence to lipophilic substrates. Lactonase activity is implicated in the antiatherogenic property of PON1 by reducing the oxidative function of LDL and macrophages [17]. It has been demonstrated that lactonase activity occurs even if it is not connected to the HDL environment [17][25].
PON1 is also a factor that is implicated in the regulation of blood pressure, and the main mechanism is controlled by 5,6-epoxyeicosateroic acid, which might also affect the salt-sensitive, high-pressure mechanism that involves the RAAS pathway and is efficiently targeted in the treatment of high blood pressure [26]. Another study demonstrated the substitutive effect of 5,6-δ-DHTL (5,6-δ-dihydroxyeicosatrienoic lactone) acid and PON1 regarding the vasodilatation mediated by Ca2+ in mice [27]. The activity of PON1 is supposed to be present only while it is bonded to the endothelial membrane and, in consequence, endothelial dysfunction leads to the internalization and enzymatic degradation of the enzyme [28].
Paraoxonase 1 is diminished in infectious diseases, it can be used as a marker, and it can lead to lower protection against the microorganism; therefore, it can play a part in the development of antibiotic resistance. In those with severe sepsis, PON1 can be used as a marker serum suggestive of a worse prognosis. Another advantage is that PON1 can fight against endotoxins from Gram-negative bacteria [29][30]
3. PON1 Implication in Atherogenesis and Cardiovascular Diseases
HDL within normal limits is responsible for cholesterol efflux, for stimulating nitric oxide, prostacyclin (PGI2), and cyclooxygenase with their vessel protective proprieties, and for the anti-inflammatory response via paraoxonase 1 [31]. Also, HDL inhibits the factor that activates plaquettes (PAF); therefore, it plays a role in stopping platelet aggregation [32]. The bonding between HDL and PON1 is influenced by some apolipoproteins such as apoA-1 and ApoJ, which are implicated in metabolic diseases, and the bonding is promoted by scavenger receptor class B type I (SR-BI). Although HDL is necessary for its activity, PON1 is also likely to be found in chylomicrons and VLDL (very low-density lipoprotein), but in smaller concentrations [15]. MPO is a protein that is usually present in healthy people’s plasma in very low concentrations, and levels are elevated in atherosclerotic diseases like acute coronary syndrome or those with metabolic syndrome, and it affects PON1 activity, decreasing its activity [33][34]. A high MPO/PON1 ratio is representative of HDL dysfunction; MPO inactivates the function of HDL and oxidases the tyrosine residue of PON1 that is responsible for the connection with HDL; therefore, it is linked with atherosclerotic disease [33][35]. HDL extracted from those with a higher MPO/PON1 ratio does not have the same anti-inflammatory effects, and in those patients’ serum, the NF-kB pathway is activated [35]. MPO levels can be used in the assessment of the instability of the atherosclerotic plaque according to recent studies [36]. It has been found in mice models that the absence of PON1 leads to lower levels of glycerin, PUFA ratio, 3 hydroxybutyrates, and carnitin, which means lower lipolytic activity and less lipid oxidation [4]. Furthermore, this can reduce the homocysteinilation of proteins with less hcy-thiolactone, and lead to a lower risk for cardiovascular, autoimmune, and neurological disease. It has three main activities: arylesterase (enhanced by ApoA-1), lactonase, and organophosphate activity [37].
Kunutsor et al. demonstrated in a study that PON1 activity is more firmly related to HDL-C and ApoA-1 and has a linear association with CVD (cardiovascular disease). HDL-C is believed to be a stronger risk indicator for CVD than PON1, but another study shows PON1 as a better indicator in men known before with cardiovascular disease [38]. Results from a recent study show that PON1 arylesterase activity in people with coronary arterial disease is lower than in normal people [39].
One of the keys to stopping atherogenesis is to prevent low-density lipoprotein oxidation. Oxidated forms of LDL and HDL cholesterol by ROS are responsible for inflammation, and they interfere with glucosidic and lipid metabolism, especially in the Krebs cycle, glycolysis, and phospholipid metabolism. Therefore, metabolic diseases that lead to high ROS levels decrease PON1 levels and its ability to stop LDL oxidation and the activation of pro-inflammatory cells [4]. Furthermore, low PON1 activity leads to HDL dysfunction, which is responsible for more LDL oxidation and higher LDL levels, since HDL usually has protective antioxidant and anti-inflammatory properties, and its normal function is vital. High oxLDL levels increase MCP-1 and lead to atherosclerosis [4]. PON1 can hydrolyze oxidized fatty acids, triglyceride hydroperoxides, and cholesterylesters, which are implicated in atherosclerosis; therefore, this is one of the mechanisms that saves the LDL from oxidation [40]. Another mechanism is given by PON1’s ability to hydrolyze the macrophage plasma membrane surface of phospholipids when combined with HDL. This process drives n-lysophosphatidylcholine (LPC) production, which inhibits cholesterol fusion [41]. There have been studies on macrophages showing that PON1 reduces macrophage oxidative stress, indicating their role in oxidizing LDL [17]. A study including 3668 patients without acute coronary syndrome who received a coronary angiography had PON and arylesterase activity measured and re-evaluated for 3 years, showing that low levels are associated with a higher chance for major cardiovascular events [42]. Also, it is believed that PON1 activity is already diminished before the acute event, since the activity is lower within 2 h of the symptoms of myocardial infarction. Besides that, PON1 is correlated with the severity of atherosclerosis [43].
PON1 has a beneficial effect and may have implications in reducing diseases by being involved in two significant pathways: peroxisome-proliferator-activated receptor gamma (PPAR-γ) and protein kinase B/nuclear factor kappa–light chain enhancer of activated B cells (AKT/NF-kB). Growth factors and other effectors stimulate all the PON1 pathways, leading to the formation, differentiation, and apoptosis of the cells. IL-1β and TNF-α (tumor necrosis factor) reduce PON1 activity, inhibiting PPAR-α activation via NF-kB and IL-6 (interleukin-6) stimulates PON1 via AKT/NF-kB. NF-kB is a transcription factor and plays a role in the expression of pro-inflammation [44].
During a pro-inflammatory phase with increased IL-6, IL-1 β (interleukin-1 β), TNF-α, serum amyloid A (SAA), and reduced PON1, the mRNA inhibitor of NF-kB translocation to the nucleus or the transient overexpression of IkB partially restored PON1 mRNA levels [45]. PPAR antagonists are widely used drugs in medicine; fibrates as hypolipidemic drugs and thiazolidinediones are used in treating diabetes [46]. The effect of fibrates on PON1 has contradictory results depending on the type, dose, and length of treatment. Fenofibrate increased PON1 activity in people with high levels of cholesterol and ischemic heart disease, while bezafibrate and gemfibrozil for 8 weeks did not have a direct effect on PON1 [45][47]. It is well known that CRP is one of the best markers for inflammation, and Mackness et al. also investigated the PON1: CRP ratio for its clinical relevance to coronary heart disease. The CRP is higher in non-diabetic subjects with coronary heart disease than in people with diabetes, and PON1 is the most reduced, but further investigations are needed to assess if those are the best markers [48].
IL-6, a cytokine known for its implication in atherogenesis, is responsible for increasing the function and protein level of PON1, but this does not apply to IL-1 β or TNF- α in this study. The mechanism behind this process is observed at the transcriptional level by the power of IL-6 to activate the JAK/STAT 3 (Janus kinase) signal pathway and the activation of NF-kB. Therefore, IL-6 helps the binding between NF-kB and the PON1-specific response sequence, and this effect was annulled by inhibiting the IL-6-induced PON1 gene expression in an experimental study with NF-kB inhibitors [37]. On the other hand, another study suggests that IL-6 plays an important role in PON1 activity, showing that the long-term regulation of PON1 by IL-6 was detrimental [49].
Studies on mice showed higher levels of the aortic adhesion molecules P-selectin and ICAM1 mRNA, greater leukocyte adhesion, and higher levels of aortic superoxide, supporting the idea that a lack of PON1 means oxidative stress and the beginning of atherosclerosis. Also, there was less cellular HDL binding. Additional studies with mice models using leptin and LDL (low-density lipoprotein) receptor deficiency, stimulating metabolic syndrome, have led to atherosclerosis, dyslipidemia, and obesity. In this study, an overexpression of PON1 resulted in decreased levels of oxidized LDL and plaque volume [45].
Interestingly, a newer study has found that PON1 and its arylesterase activity were diminished in patients with atrial fibrillation. Still, atrial fibrillation is responsible for the changes in PON1, but not for its activity. The explanation for this finding can be that atrial fibrillation’s risk factors are the same that lead to lower PON1 levels [50].
Reduced PON1 levels were found in patients with carotid stenosis who were symptomatic in comparison to those who did not have any symptoms. Still, the levels were not associated with cholesterol or triglyceride levels, which can be explained because complicated plaques contained more oxidized LDL, and symptomatic patients were older. It is known that PON1 levels are reduced with aging [51]. A recent study on patients with non-ST-segment elevation myocardial infarction showed that diminished PON1 levels were found in those who had an increased risk of death [52].
Recent studies determined that PON genes are not only responsible for protection against atherosclerotic cardiovascular diseases, but also against those that are not atherogenic (Figure 2). They protect against hypertrophy of the heart, a mechanism used for compensation in hypertensive patients but that can lead to heart failure over time [53][54].
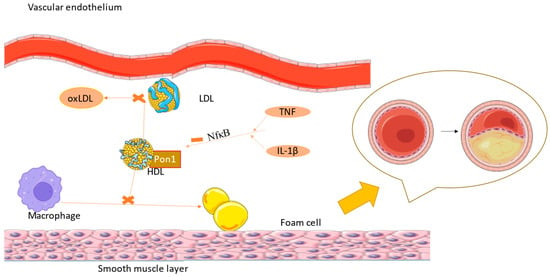
Figure 2. IL-1 β and TNF-α inhibit PON1 function via the NF-kB pathway, leading to higher levels of oxLDL, with atherosclerosis being the consequence (the figure was composed using Servier Medical Art templates and PowerPoint, Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/) Accessed on 23 February 2024).
4. PON1 and Diabetes
Type 2 diabetes is associated with increased oxidation with thrombotic results such as hyperaggregability [55]. The effects on glucose metabolism have been studied, finding that PON1 affects insulin sensitivity and glucose tolerance, and that the abnormal PON1 function leads to insulin resistance [15]. The relationship between them is mutual because polymorphism can be a reason for the installation of diabetes and after the onset, PON1 activity will be much lower [56]. The malfunction of PON1 activity has been found in both diabetes mellitus type 1 and type 2. These results are also important for cardiovascular diseases, since diabetes is one of the most important risk factors. Diabetes also has a role in lipidic metabolism, being responsible for increased triglycerides and LDL cholesterol levels and decreased HDL levels. If the triglyceride part of HDL is higher than normal, then the chances of plaque apparition in patients with diabetes are higher [57]. It is well known that HDL plays a role in glucose metabolism by promoting insulin release from pancreatic cells and the conservation of glucose in skeletal muscle [4]. In type 1 diabetes, Vaisar et al. found that high PON1 levels and moderately increased HDL values were responsible for vascular protection without taking into consideration the rest of the lipid metabolism’s markers [58].
Regarding the mechanism, it is believed that increased levels of diacylglycerol caused by high levels of glucose can cause PON1 gene transactivation via the PKC-mediated pathway [41]. The body’s response to insulin by GLUT4 expression is increased by PON1, but PON1 levels are independently reduced by type 2 diabetes, with a decreasing trend as diabetes progresses [59][60]. In diabetic patients, PON1 activity is lower, and it can go even lower in those who are also suffering from microvascular complications of diabetes, leading further to a higher chance of developing atherosclerosis [33]. Studies suggest that low PON1 levels in diabetes are one of the causes of retinopathy and even nephropathy. When insulin resistance occurs, first there are no significant changes in PON1, showing the importance of an early diagnosis and of lifestyle changes and treatment in the first phase [61]. An interesting new study showed that control females had higher PON1 activity than men, but when the comparison between type 2 diabetic patients was made, they had lower levels than men [62].
5. PON1 and Stroke
Over the years, stroke has become the second cause of death and disability worldwide. More than 15 million people are affected by this disease, defined as an acute syndrome with a focal neurological deficit due to vascular injury located in the central nervous system. The most prevalent type of stroke is ischemic, which comprises approximately 85–90% of all cerebral infarcts. Ischemic strokes are caused mostly by arteriolosclerosis located in the small cerebral vessels; another cause could be a cardioembolism or athero-thromboembolism located in large arteries [63]. Studies of stroke etiology showed an interaction between non-modifiable risk factors (genetic) and modifiable risk factors (environmental factors). Regarding the non-modifiable risk factors, numerous genes are linked to cholesterol metabolism, inflammation, and coagulation. Some genes are involved in the etiology of ischemic stroke. Studies have shown that the genetic variations in PON1 can predict the risk of ischemic stroke and modulate the hydrolyzing effect of PON1 over LDL oxidized phospholipids [64][65]. Although the predictive value of PON1 has not been clearly established yet, compared with healthy people, those who had an acute ischemic stroke had lower HDL levels and diminished PON1 activity, with the 107 T allele being responsible for a higher risk of developing an acute stroke [66]. Also, the rs854560 polymorphism is more prone to ischemic stroke [67]. Carotid atherosclerosis can be a cause of stroke, and PON1 is implicated in intima media thickness, but some studies showed a link between them only in the 55LL genotype. Another study found that the rs662 polymorphism is associated with the severity of carotid stenosis [68][69]. The enzyme PON1 is supposed, in various studies, to be one of the most important markers, along with HDL cholesterol, to limit LDL cholesterol oxidation, preventing first atherosclerosis and second stroke [65][68][70]. Further studies are required to establish a relation between PON1 activity levels and the prognosis of patients with ischemic stroke, and this relationship may have clinical and therapeutic importance in neurological patients [65][70].
6. PON1 and Other Diseases
Obesity has also been found to be involved in lower PON1 activity and higher levels of HDL and LDL hydroperoxides, meaning more oxidative stress. Also, leptin levels are indirectly proportional to PON1 arylesterase activity, while adipokines are positively correlated with arylesterase PON1 activity [4]. Obesity leads to a higher chance of developing cardiovascular disease and moreover, the body index is directly related to PON1 [61]. One reason can be based on the adipocytes, since they not only store the lipids but also produce pro-inflammatory cytokines and adipokines that inhibit liver PON1 production [71].
Uremic components can interfere with HDL structure, and since in renal disease their elimination is reduced, it will lead to lower PON1 activity. PON3 is also affected by chronic kidney disease. Kotur et al. also studied PON1 levels in COPD patients, who had lower PON1 levels than the control group. Also, the same result has been found in sarcoidosis [72]. A recent review showed that PON1 was lower in the serum of asthmatic patients, but it seemed that it was not correlated with the severity of the disease [73]. The same group investigated its activity in psoriasis with strongly reduced PON1 levels, but no differences were found in arylesterase activity [74]. PCOS can be associated with PON1 deficiency; however, mostly depending on the polymorphism, in Kashmiri women only, 108C/T, but not 55L/M genes, were associated with PCOS, increasing the chance of developing metabolic syndrome and insulin resistance [75]. Nonalcoholic fatty liver disease (NAFLD) is a chronic liver condition associated with excessive fat that builds up in the liver, and it can progress into NASH (nonalcoholic steatohepatitis) and finally to cirrhosis or liver cancer. Kotani et al. proved that PON1 activity is diminished in NAFLD, and they suggested that it can be used as a marker for it [76].
7. PON1 and Therapy
Statins, inhibitors of hydroxymethylglutaryl-coenzyme A reductase, are the main choices for lowering cholesterol levels, but are also implicated in the stabilization of pre-existent plaques, protection against oxLDL, increased NO effect, and upregulation of eNOS. Also, they block the NF-kB pathway responsible for inflammation [2].
PON1 activity’s response to statin therapy has been studied, with two different outcomes: one study suggested increasing activity, while another study with simvastatin suggested no changes [17]. Deakin et al. studied simvastatin in patients, measuring total cholesterol, LDL, HDL, triglycerides, PON1 concentration, PON1 activity ARE, PON1 activity PONb, specific activity ARE, and specific activity PONb, with higher activity for paraoxon and phenylacetate hydrolysis, both of them being associated with a higher serum concentration of PON1 [77]. Usually, in studies, PON1 activity is measured using paraoxonase activity and arylesterase activity, since AREase is not affected as much as others by genetic polymorphism, so it has a minimal interindividual. Also, it is a reliable marker for measuring PON1 levels and activity [17]. Another study on simvastatin conducted by Kumar showed that LDL levels were diminished with no influence on HDL, but after 4 months of treatment, PON1 levels rose [78]. As far as PON1 polymorphism is considered, studies showed a positive effect on HDL with pravastatin in an R allele carrier than QQ homozygous, and better levels in RR homozygous during simvastatin therapy. DE Souza et al. had a different outcome with RR carriers who did not reach the HDL target as easily as those with the Q allele [79]. Clopidogrel, an antiplatelet therapy, used in the prevention and treatment of patients who undergo coronary angiography with stent implantation, has been a controversial study. Some studies suggest that the PON1 QQ192 genotype might be responsible for some resistance to clopidogrel because this genotype has been associated with stenosis intrastent, but others found no connection [80]. Ticagrelor, used more and more frequently in the past few years in patients with coronary stents, has had auspicious results regarding its effectiveness in protection against another ischemic event, and more importantly, it has the ability to induce PON1, leading to higher serum levels in comparison to clopidogrel [81]. Acetylsalicylic acid, a highly used drug in cardiovascular medicine, known for its protective antithrombotic and anti-inflammatory effects, had a positive impact on PON1 in studies on people with an atherosclerotic coronary [82]. Ezetimibe, a drug used in combination with statin therapy in patients whose target cholesterol level cannot obtained with statins alone, is used for stopping cholesterol absorption and was studied in 2010, showing that antioxidant parameters such as PON1 and TAC levels were increased substantially [83].
8. PON1 and Lifestyle
Diet plays a crucial role, since increased lipid intake leads to inflammation and higher levels of ROS (reactive oxygen species), pro-inflammatory cytokines, IL-1, and IL-6, all of which result in lower PON1 liver secretion and decreased PPAR-γ. On the other hand, a high-sucrose diet has had controversial results. In vivo studies demonstrated that high sucrose intake is responsible for hyperlipemia and increased oxidative stress, also suggesting lower PON1 activity [4]. Another study has found that a high-sucrose diet leads to high PON1 activity after 3–5 weeks [84]. The Mediterranean diet, recommended for the prevention of cardiovascular diseases, has resulted in higher postprandial PON1 activity, which might be the result of using extra virgin olive oil [4]. Studies on mice on an atherogenic diet demonstrated no changes in gene expression, but lower activity of PON1 [45]. Meals cooked in deep-fried oil lowered HDL and PON1 4 h after their consumption, and the effects lasted approximately 8 h [45]. Extra virgin olive oil is recommended to be used instead of sunflower oil because studies have shown numerous beneficial effects like reducing inflammation (also CRP and IL-6), oxidative stress, and increasing the levels and function of HDL [18]. As expected, since cigarette smoking increases oxidative stress, in smokers there was also lower PON1 activity [85][86]. As far as alcohol consumption is concerned, the results are interesting. A study showed that chronic light alcohol intake raises PON1, while heavy drinking leads to lower PON1 expression. This explication comes from the fact that ethanol, by stimulating PKC, will raise PON1, but heavy consumption leads to PKC overexpression, which will inhibit PON1 [41][87]. Also, red wine, which contains antioxidant components, has led to higher HDL and lower LDL levels, mainly due the effect of the alcohol, as described, and the effect of resveratrol on PPAR-γ receptors [41][45]. PON1 is also modulated by physical activity, with lower levels in people who are sedentary and obese. Still, it has also been found that 192QQ subjects had higher levels compared to R carriers, meaning it is also dependent on some genes [45][88][89]. The main functions of PON1 are included in Table 1. Aging is associated with a decline in cell function, with a higher susceptibility to increased oxidative stress and inflammation; therefore, lower levels of PON1 are expected as well [11].
Table 1. PON1 main functions.
| 1. Preservation of HDL by fighting against its oxidation [19] -higher cholesterol efflux [19] |
| 2. Preventing LDL oxidation [19] -less oxidized lipids, which are responsible for inflammation [45] -lower LDL levels [19] |
| 3. Protects against insulin resistance [4] |
| 4. Ameliorates effects of oxidized LDL [19] -↓ inflammatory and cytotoxic oxidized phospholipids [19] -↓ LDL uptake by macrophages [19] -↓ monocyte transmigration induced by oxidized LDL [19] |
| 5. Atheroprotective [17] -decreases lipid peroxides in atherosclerotic lesions [19] -reduces macrophage oxidative stress and the ability of macrophages to oxidize LDL [17] -contributes to the metabolism of homocysteine thiolactones [17] -prevents the oxidative inactivation of lecithin cholesterol acyltransferase [17] -reduces monocyte–macrophage inflammatory response [17] -reduces foam cell formation [68] -restores normal endothelial function [68] |
| 6. Antiapoptotic [72] |
| 7. Vasodilative [72] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms25052962
References
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069.
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967.
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321.
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Macedo Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. IJMS 2019, 20, 4049.
- Schiffrin, E.L.; Park, J.B.; Pu, Q. Effect of Crossing over Hypertensive Patients from a Beta-Blocker to an Angiotensin Receptor Antagonist on Resistance Artery Structure and on Endothelial Function. J. Hypertens. 2002, 20, 71–78.
- Husain, K. Inflammation, Oxidative Stress and Renin Angiotensin System in Atherosclerosis. WJBC 2015, 6, 209.
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636.
- Landmesser, U.; Harrison, D.G. Oxidant Stress as a Marker for Cardiovascular Events: Ox Marks the Spot. Circulation 2001, 104, 2638–2640.
- Galley, H.F.; Webster, N.R. Physiology of the Endothelium. Br. J. Anaesth. 2004, 93, 105–113.
- Best, P.J.M.; Lerman, A. Endothelin in Cardiovascular Disease: From Atherosclerosis to Heart Failure. J. Cardiovasc. Pharmacol. 2000, 35, S61–S63.
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vasc. Pharmacol. 2018, 100, 1–19.
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent Insights into the Cellular Biology of Atherosclerosis. J. Cell Biol. 2015, 209, 13–22.
- Sandoo, A.; Veldhuijzen Van Zanten, J.J.C.S.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. TOCMJ 2010, 4, 302–312.
- Balta, S. Endothelial Dysfunction and Inflammatory Markers of Vascular Disease. CVP 2020, 19, 243–249.
- Arab, Z.N.; Khayatan, D.; Razavi, S.M.; Zare, K.; Kheradkhah, E.; Momtaz, S.; Ferretti, G.; Bacchetti, T.; Sathyapalan, T.; Emami, S.A.; et al. Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases. Antioxidants 2022, 11, 1273.
- Taler-Verčič, A.; Goličnik, M.; Bavec, A. The Structure and Function of Paraoxonase-1 and Its Comparison to Paraoxonase-2 and -3. Molecules 2020, 25, 5980.
- Mahrooz, A.; Mackness, M.; Bagheri, A.; Ghaffari-Cherati, M.; Masoumi, P. The Epigenetic Regulation of Paraoxonase 1 (PON1) as an Important Enzyme in HDL Function: The Missing Link between Environmental and Genetic Regulation. Clin. Biochem. 2019, 73, 1–10.
- Khalil, A.; Fulop, T.; Berrougui, H. Role of Paraoxonase1 in the Regulation of High-Density Lipoprotein Functionality and in Cardiovascular Protection. Antioxid. Redox Signal. 2021, 34, 191–200.
- Draganov, D.I.; La Du, B.N. Pharmacogenetics of Paraoxonases: A Brief Review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 78–88.
- Priyanka, K.; Singh, S.; Gill, K. Paraoxonase 3: Structure and Its Role in Pathophysiology of Coronary Artery Disease. Biomolecules 2019, 9, 817.
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What Are Their Functions? Chem. Biol. Interact. 2016, 259, 51–62.
- Mohamed, R.H.; Mohamed, R.H.; Karam, R.A.; Abd El-Aziz, T.A. The Relationship between Paraoxonase1-192 Polymorphism and Activity with Coronary Artery Disease. Clin. Biochem. 2010, 43, 553–558.
- Soflaei, S.S.; Baktashian, M.; Moghaddam, K.H.; Saberi-Karimian, M.; Kosari, N.; Hashemi, S.M.; Mouhebati, M.; Amini, M.; Dehghani, M.; Esmaily, H.; et al. Association of Paraoxonase-1 Genotype and Phenotype with Angiogram Positive Coronary Artery Disease. Arq. Bras. Cardiol. 2022, 119, 593–601.
- Ashiq, S.; Ashiq, K. The Role of Paraoxonase 1 (PON1) Gene Polymorphisms in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Biochem. Genet. 2021, 59, 919–939.
- Deakin, S.P.; Bioletto, S.; Bochaton-Piallat, M.-L.; James, R.W. HDL-Associated Paraoxonase-1 Can Redistribute to Cell Membranes and Influence Sensitivity to Oxidative Stress. Free Radic. Biol. Med. 2011, 50, 102–109.
- Gamliel-Lazarovich, A.; Abassi, Z.; Khatib, S.; Tavori, H.; Vaya, J.; Aviram, M.; Keidar, S. Paraoxonase1 Deficiency in Mice Is Associated with Hypotension and Increased Levels of 5,6-Epoxyeicosatrienoic Acid. Atherosclerosis 2012, 222, 92–98.
- Gilad, D.; Atiya, S.; Mozes-Autmazgin, Z.; Ben-Shushan, R.S.; Ben-David, R.; Amram, E.; Tamir, S.; Chuyun, D.; Szuchman-Sapir, A. Paraoxonase 1 in Endothelial Cells Impairs Vasodilation Induced by Arachidonic Acid Lactone Metabolite. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1864, 386–393.
- Raz, B.-D.; Dimitry, C.; Andrea, S.-S. The Uptake Mechanism and Intracellular Fate of Paraoxonase-1 in Endothelial Cells. Free Radic. Biol. Med. 2020, 153, 26–33.
- Camps, J.; Iftimie, S.; García-Heredia, A.; Castro, A.; Joven, J. Paraoxonases and Infectious Diseases. Clin. Biochem. 2017, 50, 804–811.
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and Atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967.
- Park, S.; Mathis, K.W.; Lee, I.K. The Physiological Roles of Apolipoprotein J/Clusterin in Metabolic and Cardiovascular Diseases. Rev. Endocr. Metab. Disord. 2014, 15, 45–53.
- Kowalska, K.; Socha, E.; Milnerowicz, H. Review: The Role of Paraoxonase in Cardiovascular Diseases. Ann. Clin. Lab. Sci. 2015, 45, 226–233.
- Jeelani, H.; Tabassum, N.; Afroze, D.; Rashid, F. Association of Paraoxonase1 Enzyme and Its Genetic Single Nucleotide Polymorphisms with Cardio-Metabolic and Neurodegenerative Diseases. Gene Rep. 2020, 20, 100775.
- Variji, A.; Shokri, Y.; Fallahpour, S.; Zargari, M.; Bagheri, B.; Abediankenari, S.; Alizadeh, A.; Mahrooz, A. The Combined Utility of Myeloperoxidase (MPO) and Paraoxonase 1 (PON1) as Two Important HDL-Associated Enzymes in Coronary Artery Disease: Which Has a Stronger Predictive Role? Atherosclerosis 2019, 280, 7–13.
- Bacchetti, T.; Ferretti, G.; Carbone, F.; Ministrini, S.; Montecucco, F.; Jamialahmadi, T.; Sahebkar, A. Dysfunctional High-Density Lipoprotein: The Role of Myeloperoxidase and Paraoxonase-1. CMC 2021, 28, 2842–2850.
- Yunoki, K.; Naruko, T.; Inaba, M.; Inoue, T.; Nakagawa, M.; Sugioka, K.; Ohsawa, M.; Iwasa, Y.; Komatsu, R.; Itoh, A.; et al. Gender-Specific Correlation between Plasma Myeloperoxidase Levels and Serum High-Density Lipoprotein-Associated Paraoxonase-1 Levels in Patients with Stable and Unstable Coronary Artery Disease. Atherosclerosis 2013, 231, 308–314.
- Cheng, C.-C.; Hsueh, C.-M.; Chen, C.-Y.; Chen, T.-H.; Hsu, S.-L. Interleukin-6 Upregulates Paraoxonase 1 Gene Expression via an AKT/NF-κB-Dependent Pathway. Biochem. Biophys. Res. Commun. 2013, 437, 55–61.
- Kunutsor, S.K.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum Paraoxonase-1 Activity and Risk of Incident Cardiovascular Disease: The PREVEND Study and Meta-Analysis of Prospective Population Studies. Atherosclerosis 2016, 245, 143–154.
- Zuin, M.; Trentini, A.; Marsillach, J.; D’Amuri, A.; Bosi, C.; Roncon, L.; Passaro, A.; Zuliani, G.; Mackness, M.; Cervellati, C. Paraoxonase-1 (PON-1) Arylesterase Activity Levels in Patients with Coronary Artery Disease: A Meta-Analysis. Dis. Markers 2022, 2022, 4264314.
- Martini, D.; Del Bo’, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of Polyphenols and Polyphenol-Rich Foods in the Modulation of PON1 Activity and Expression. J. Nutr. Biochem. 2017, 48, 1–8.
- Kunachowicz, D.; Ściskalska, M.; Kepinska, M. Modulatory Effect of Lifestyle-Related, Environmental and Genetic Factors on Paraoxonase-1 Activity: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2813.
- Mackness, M.; Mackness, B. Human Paraoxonase-1 (PON1): Gene Structure and Expression, Promiscuous Activities and Multiple Physiological Roles. Gene 2015, 567, 12–21.
- Zhao, Y.; Ma, Y.; Fang, Y.; Liu, L.; Wu, S.; Fu, D.; Wang, X. Association between PON1 Activity and Coronary Heart Disease Risk: A Meta-Analysis Based on 43 Studies. Mol. Genet. Metab. 2012, 105, 141–148.
- Bacchetti, T.; Ferretti, G.; Sahebkar, A. The Role of Paraoxonase in Cancer. Semin. Cancer Biol. 2019, 56, 72–86.
- Précourt, L.-P.; Amre, D.; Denis, M.-C.; Lavoie, J.-C.; Delvin, E.; Seidman, E.; Levy, E. The Three-Gene Paraoxonase Family: Physiologic Roles, Actions and Regulation. Atherosclerosis 2011, 214, 20–36.
- Bełtowski, J.; Wójcicka, G.; Mydlarczyk, M.; Jamroz, A. The Effect of Peroxisome Proliferator-Activated Receptors Alpha (PPARalpha) Agonist, Fenofibrate, on Lipid Peroxidation, Total Antioxidant Capacity, and Plasma Paraoxonase 1 (PON 1) Activity. J. Physiol. Pharmacol. 2002, 53, 463–475.
- Yesilbursa, D.; Serdar, A.; Saltan, Y.; Serdar, Z.; Heper, Y.; Guclu, S.; Cordan, J. The Effect of Fenofibrate on Serum Paraoxonase Activity and Inflammatory Markers in Patients with Combined Hyperlipidemia. Kardiol. Pol. 2005, 62, 526–530.
- Mackness, B.; Hine, D.; McElduff, P.; Mackness, M. High C-Reactive Protein and Low Paraoxonase1 in Diabetes as Risk Factors for Coronary Heart Disease. Atherosclerosis 2006, 186, 396–401.
- Van Lenten, B.J.; Wagner, A.C.; Navab, M.; Fogelman, A.M. Oxidized Phospholipids Induce Changes in Hepatic Paraoxonase and ApoJ but Not Monocyte Chemoattractant Protein-1 via Interleukin-6. J. Biol. Chem. 2001, 276, 1923–1929.
- Istratoaie, S.; Boroş, B.; Vesa, Ş.C.; Maria Pop, R.; Cismaru, G.; Pop, D.; Vasile Milaciu, M.; Ciumărnean, L.; Văcăraş, V.; Dana Buzoianu, A. Paraoxonase 1 and Atrial Fibrillation: Is There a Relationship? Medicine 2022, 101, e31553.
- Lioudaki, S.; Verikokos, C.; Kouraklis, G.; Kontopodis, N.; Markakis, G.; Ioannou, C.; Daskalopoulou, A.; Perrea, D.; Klonaris, C. Paraoxonase-1 and Symptomatic Status in Carotid Artery Disease. Ann. Vasc. Surg. 2020, 64, 355–360.
- Leocádio, P.C.L.; Goulart, A.C.; Santos, I.S.; Lotufo, P.A.; Bensenor, I.M.; Alvarez-Leite, J.I. Lower Paraoxonase 1 Paraoxonase Activity Is Associated with a Worse Prognosis in Patients with Non-ST-Segment Elevation Myocardial Infarction in Long-Term Follow-Up. Coron. Artery Dis. 2022, 33, 515–522.
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and Atherosclerosis-Related Cardiovascular Diseases. Biochimie 2017, 132, 19–27.
- Pei, J.-F.; Yan, Y.-F.; Tang, X.; Zhang, Y.; Cui, S.-S.; Zhang, Z.-Q.; Chen, H.-Z.; Liu, D.-P. Human Paraoxonase Gene Cluster Overexpression Alleviates Angiotensin II-Induced Cardiac Hypertrophy in Mice. Sci. China Life Sci. 2016, 59, 1115–1122.
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A Review of the Mechanisms and Effectiveness of Dietary Polyphenols in Reducing Oxidative Stress and Thrombotic Risk. J. Hum. Nutr. Diet. 2014, 27, 1–21.
- Adiga, U.; Banawalikar, N.; Menambath, D.T. Association of Paraoxonase 1 Activity and Insulin Resistance Models in Type 2 Diabetes Mellitus: Cross-Sectional Study. J. Chin. Med. Assoc. 2022, 85, 77.
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of Paraoxonase 1 (PON1) as an Antioxidant and Antiatherogenic Enzyme in the Cardiovascular Complications of Type 2 Diabetes: Genotypic and Phenotypic Evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067.
- Vaisar, T.; Kanter, J.E.; Wimberger, J.; Irwin, A.D.; Gauthier, J.; Wolfson, E.; Bahnam, V.; Wu, I.-H.; Shah, H.; Keenan, H.A.; et al. High Concentration of Medium-Sized HDL Particles and Enrichment in HDL Paraoxonase 1 Associate With Protection From Vascular Complications in People With Long-Standing Type 1 Diabetes. Diabetes Care 2020, 43, 178–186.
- Nessler, K.; Grzybczak, R.; Nessler, M.; Zalewski, J.; Gajos, G.; Windak, A. Associations between Myeloperoxidase and Paraoxonase-1 and Type 2 Diabetes in Patients with Ischemic Heart Disease. BMC Cardiovasc. Disord. 2022, 22, 521.
- Koren-Gluzer, M.; Aviram, M.; Hayek, T. Paraoxonase1 (PON1) Reduces Insulin Resistance in Mice Fed a High-Fat Diet, and Promotes GLUT4 Overexpression in Myocytes, via the IRS-1/Akt Pathway. Atherosclerosis 2013, 229, 71–78.
- Kota, S.K.; Meher, L.K.; Kota, S.K.; Jammula, S.; Krishna, S.V.S.; Modi, K.D. Implications of Serum Paraoxonase Activity in Obesity, Diabetes Mellitus, and Dyslipidemia. Indian J. Endocrinol. Metab. 2013, 17, 402–412.
- Dada, A.O.; Ikpegbu, U.A.; Okunowo, L.O.; Ajibare, A.O.; Adekiitan, M.E.; Shasore, H.O. Plasma Paraoxonase-1 Activity Levels in Patients with Type 2 Diabetes Mellitus in Lagos State University Teaching Hospital, Lagos, Southwest Nigeria: A Cross-Sectional Study. Pan Afr. Med. J. 2023, 45, 40.
- Murphy, S.J.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566.
- Pan, Y.; He, B.; Sun, H.; Xu, T.; Pan, B.; Wang, S.; Mei, Y. Susceptibility of PON1/PON2 Genetic Variations to Ischemic Stroke Risk in a Chinese Han Population. PGPM 2020, 13, 563–570.
- Xu, Y.; Wang, K.; Wang, Q.; Ma, Y.; Liu, X. The Antioxidant Enzyme PON1: A Potential Prognostic Predictor of Acute Ischemic Stroke. Oxidative Med. Cell. Longev. 2021, 2021, e6677111.
- Goswami, B.; Tayal, D.; Gupta, N.; Mallika, V. Paraoxonase: A Multifaceted Biomolecule. Clin. Chim. Acta 2009, 410, 1–12.
- Zeng, Q.; Zeng, J. A Meta-Analysis on Relationship between Paraoxonase 1 Polymorphisms and Atherosclerotic Cardiovascular Diseases. Life Sci. 2019, 232, 116646.
- Tajbakhsh, A.; Rezaee, M.; Rivandi, M.; Forouzanfar, F.; Afzaljavan, F.; Pasdar, A. Paraoxonase 1 (PON1) and Stroke; the Dilemma of Genetic Variation. Clin. Biochem. 2017, 50, 1298–1305.
- Sun, J.; Wang, L.; Yang, Q.; Zhou, T.; Ding, X.; Yang, K.; Zhou, Z. The Association of Paraoxonase-1 Polymorphism with Carotid Artery Stenosis among Elderly Chinese Population. Oxid. Med. Cell. Longev. 2020, 2020, 3084120.
- Zhu, H.; Zhao, T.; Liu, J. Role of Paraoxonase 1 Activity and Oxidative/Antioxidative Stress Markers in Patients with Acute Cerebral Infarction. Clin. Lab. 2018, 64, 180201.
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22.
- Kotur-Stevuljević, J.; Vekić, J.; Stefanović, A.; Zeljković, A.; Ninić, A.; Ivanišević, J.; Miljković, M.; Sopić, M.; Munjas, J.; Mihajlović, M.; et al. Paraoxonase 1 and Atherosclerosis-related Diseases. BioFactors 2020, 46, 193–205.
- Bassu, S.; Mangoni, A.A.; Argiolas, D.; Carru, C.; Pirina, P.; Fois, A.G.; Zinellu, A. A Systematic Review and Meta-Analysis of Paraoxonase-1 Activity in Asthma. Clin. Exp. Med. 2023, 23, 1067–1074.
- Bassu, S.; Mangoni, A.A.; Satta, R.; Argiolas, D.; Carru, C.; Zinellu, A. Paraoxonase and Arylesterase Activity of Serum PON-1 Enzyme in Psoriatic Patients: A Systematic Review and Meta-Analysis. Clin. Exp. Med. 2023, 23, 301–311.
- Jeelani, H.; Ganie, M.A.; Amin, S.; Fatima, Q.; Kawa, I.A.; Manzoor, S.; Parvez, T.; Ahmad, D.N.; Rashid, F. Effect of Paraoxonase1 (PON1) Gene Polymorphisms on PON1 Activity, HDL, LDL and MDA Levels in Women with Polycystic Ovary Syndrome (PCOS): A Case-Control Study. Meta Gene 2019, 20, 100552.
- Kotani, K.; Watanabe, J.; Miura, K.; Gugliucci, A. Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Molecules 2021, 26, 2323.
- Deakin, S.; Leviev, I.; Guernier, S.; James, R.W. Simvastatin Modulates Expression of the PON1 Gene and Increases Serum Paraoxonase: A Role for Sterol Regulatory Element–Binding Protein-2. ATVB 2003, 23, 2083–2089.
- Kumar, A. Effect of Simvastatin on Paraoxonase 1 (PON1) Activity and Oxidative Stress. Asian Pac. J. Trop. Med. 2010, 3, 310–314.
- De Souza, J.A.; Menin, A.; Lima, L.O.; Smiderle, L.; Hutz, M.H.; Van Der Sand, C.R.; Van Der Sand, L.C.; Ferreira, M.E.W.; Pires, R.C.; Almeida, S.; et al. PON1 Polymorphisms Are Predictors of Ability to Attain HDL-C Goals in Statin-Treated Patients. Clin. Biochem. 2015, 48, 1039–1044.
- Akram, N.; Mustafa, G.; Hanif, A.A.; Tawwab, S.; Hussain, S.; Kaul, H.; Mohsin, S. Cytochrome 2C19 and Paraoxonase-1 Polymorphisms and Clopidogrel Resistance in Ischemic Heart Disease Patients. Per. Med. 2019, 16, 379–386.
- Halim, H.; Pinkaew, D.; Chunhacha, P.; Sinthujaroen, P.; Thiagarajan, P.; Fujise, K. Ticagrelor Induces Paraoxonase-1 (PON1) and Better Protects Hypercholesterolemic Mice against Atherosclerosis Compared to Clopidogrel. PLoS ONE 2019, 14, e0218934.
- Blatter-Garin, M.C.; Kalix, B.; De Pree, S.; James, R.W. Aspirin Use Is Associated with Higher Serum Concentrations of the Anti-Oxidant Enzyme, Paraoxonase-1. Diabetologia 2003, 46, 594–595.
- Turfaner, N.; Uzun, H.; Balci, H.; Ercan, M.A.; Karter, Y.H.; Caner, M.; Sipahioglu, F.; Genc, H. Ezetimibe Therapy and Its Influence on Oxidative Stress and Fibrinolytic Activity. South. Med. J. 2010, 103, 428–433.
- Macan, M.; Vrkić, N.; Vrdoljak, A.L.; Radić, B.; Bradamante, V. Effects of High Sucrose Diet, Gemfibrozil, and Their Combination on Plasma Paraoxonase 1 Activity and Lipid Levels in Rats. Acta Biochim. Pol. 2010, 57, 321–326.
- Mouhamed, D.H.; Ezzaher, A.; Araoud, M.; Neffati, F.; Douki, W.; Fadhel Najjar, M. Etude de l’activité de La Paraoxonase 1 (PON1) et Du Profil Lipidique Dans Une Population de Fumeurs Tunisiens. Ann. De Biol. Clin. 2010, 68, 143–147.
- Otocka-Kmiecik, A. Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients 2022, 14, 2842.
- Rao, M.N.; Marmillot, P.; Gong, M.; Palmer, D.A.; Seeff, L.B.; Strader, D.B.; Lakshman, M.R. Light, but Not Heavy Alcohol Drinking, Stimulates Paraoxonase by Upregulating Liver mRNA in Rats and Humans. Metabolism 2003, 52, 1287–1294.
- Seres, I.; Bajnok, L.; Harangi, M.; Sztanek, F.; Koncsos, P.; Paragh, G. Alteration of PON1 Activity in Adult and Childhood Obesity and Its Relation to Adipokine Levels. In Paraoxonases in Inflammation, Infection, and Toxicology; Reddy, S.T., Ed.; Advances in Experimental Medicine and Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 660, pp. 129–142. ISBN 978-1-60761-349-7.
- Cakmak, A.; Zeyrek, D.; Atas, A.; Erel, O. Paraoxonase Activity in Athletic Adolescents. Pediatr. Exerc. Sci. 2010, 22, 93–104.
This entry is offline, you can click here to edit this entry!
