Forests represent a vital natural resource and play a crucial role in climate regulation and maintaining biodiversity. However, the growth and development of forest trees are increasingly challenged by rising environmental pressures, particularly detrimental abiotic stressors. To address these challenges, genetic transformation technologies have emerged as effective solutions. Despite various difficulties in genetic transformation for forest trees, including prolonged life cycles, genetic diversity, interspecies variations, and complex regeneration systems, significant research progress has been achieved in tree gene editing, transgenic technology, and methods for delivering exogenous molecules. These technologies have the potential to enhance tree quality, increase productivity, and improve resistance to abiotic stress. This review provides an overview of the main methods and transformation receptors in tree genetic transformation. Additionally, we summarize several novel techniques, such as nanoparticle-mediated gene transformation, advanced gene editing technology, various novel delivery carriers, and non-genetically modified protein function interference through peptide aptamer. Notably, we also place emphasis on several referable genes from forest trees and common crops, together with their potential function for improving abiotic stress responses. Through this research, we aspire to achieve sustainable utilization and conservation of tree resources, thereby providing substantial support for future livelihoods and economic development.
1. Introduction
Forests represent a vital natural resource, with their yield and quality exerting a direct influence on human life quality and economic development [
1,
2]. Traditional breeding methods are inadequate to cope with escalating environmental pressures and the intricate challenges posed by pests and diseases. Moreover, the genetic improvement of trees is constrained by issues such as long-life cycles, long generation times, late sexual maturity and limited genetic diversity [
3,
4]. Therefore, tree genetic transformation has become a focal point of intensive research. This process involves leveraging modern biotechnological approaches to introduce exogenous genes or other genetic material into plant cells, thereby altering their genetic characteristics. Genome editing technology is a potent approach for modifying, adding, or deleting genes within the genome [
5]. In recent years, cisgenics has emerged as a technology distinct from transgenics, as it excludes the introduction of genes from other species [
6]. Occasionally, gene editing techniques are employed to introduce synthetic or artificially engineered genes into host cells [
7]. These advancements have propelled significant research progress in fields such as agriculture, forestry, and environmental conservation, demonstrating vast application prospects [
8,
9].
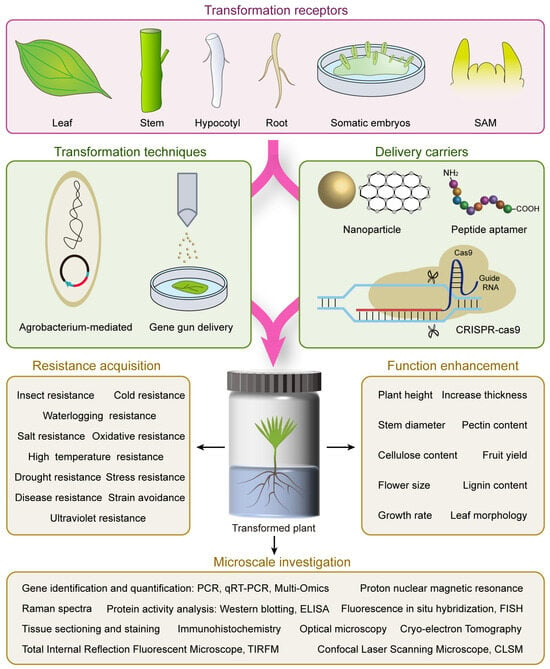
Tree genetic transformation emerges as a potent method, yielding enhancements in stress tolerance, yield augmentation, and quality enhancement (
Figure 1). For instance, the introduction of drought-tolerant genes allows trees to flourish in arid environments. Furthermore, altering tree growth patterns and lignin content can enhance salt tolerance [
10]. This review commences with a concise overview of the methods and transformation receptors utilized in tree genetic transformation and then delves into recent advances in techniques promoting transformation efficiency and success rate. We systematically summarize numerous applications of genetic transformation in improving tree stress tolerance and highlight the potential application prospects and challenges of these technologies in enhancing stress tolerance.
Figure 1. Ideograph showing common experimental procedure and transformation receptor used in forest tree genetic transformation and following investigation. The procedure mainly consists of three parts, including the selection and preparation of the transformation receptor, the utilization of appropriate transformation techniques to introduce the delivery carrier into the transformation receptor, and finally conducting stress resistance testing, functional analysis, and microscale investigations on the successfully transformed plants.
2. Tree Genetic Transformation Techniques and Transformation Receptor
With the development of molecular biology and genetic engineering techniques, tree genetic transformation techniques have significantly improved and become more refined. Currently, commonly used tree genetic transformation methods include the gene gun method and the
Agrobacterium-mediated method [
11,
12]. The choice of transformation receptor is an important factor influencing plant genetic transformation. Transformation receptors are isolated from plant tissues that can be genetically modified, such as organs, cells or protoplasts. Under controlled artificial conditions, the cultivation of transformation recipients is performed to obtain regenerated transgenic whole plants or to implement the technology for producing economically valuable products [
13]. Due to the challenges in establishing a regeneration system for trees, the options for suitable transformation receptors for tree genetic transformation are limited [
14]. Typically, tree cells or tissues are cultured and regenerated in vitro using tissue culture and regeneration techniques to obtain transformation receptors [
15].
2.1. Common Tree Genetic Transformation Techniques
The common genetic transformation systems can be categorized into two types: the gene gun and the
Agrobacterium-mediated method. In the year 1994,
GUS was successfully transferred into the anther-derived calluses of
Hevea brasiliensis by gene gun [
16]. The synthetic
CRY1Ac from
Bacillus thuringiensis has been used to transform
Pinus taeda through a gene gun. These transgenic plants exhibit high levels of resistance against
Dendrolimus punctatus Walker [
17].
Agrobacterium-mediated transformation is the preferred method for genetic transformation in trees.
Agrobacterium in soil infects wounded sites in many dicotyledonous and gymnosperm plants. It inserts T-DNA into the plant genome upon infiltrating cells, ensuring stable inheritance via meiotic division. This forms the theoretical basis for
Agrobacterium-mediated plant genetic transformation [
18].
Agrobacterium tumefaciens stands as one of the primary strains used in plant genetic transformation research. Transgenic plants were successfully generated in
Abies nephrolepis,
Pinus elliottii and
Pinus radiata through the utilization of
Agrobacterium strains including LBA4404, GV3101, and EHA105 [
18,
19,
20].
Agrobacterium rhizogenes is another commonly used type of
Agrobacterium. By using
A. rhizogenes for tree genetic transformation, it facilitates gene function studies and rapid propagation of trees [
21].
Selecting the appropriate
Agrobacterium strain is essential in the genetic transformation of tree species, as it relies on their unique genetic traits, regenerative capabilities, and responses to different strains of
Agrobacterium [
22]. The leaf disc transformation mediated by
A. tumefaciens was developed by Horsch et al. [
23]. The genetic transformation of poplar leaves can be achieved with an efficiency exceeding 80% using the leaf disc [
24]. However, based on our previous research, this method is challenging to implement in coniferous trees. Somatic embryogenesis can be employed for genetic transformation in deciduous pines such as
Pinus massoniana and
Larix gmelinii, but currently remains unattainable in
Pinus tabuliformis [
25]. We propose that the challenge of genetic transformation in
P. tabuliformis could be addressed by selecting genotypes capable of somatic embryogenesis through screening. The choice of genetic transformation receptors plays a pivotal role in the success of plant genetic transformation.
2.2. Transformation Receptor Used in Forest Tree Genetic Transformation
Due to the intrinsic factors of trees and the difficulty in establishing regeneration systems, the transformation receptors suitable for tree genetic transformation primarily include mature and immature zygotic embryos, somatic embryos, seedlings, shoot meristems, and cotyledons [
25,
26,
27]. Induced transformation receptors facilitate organ differentiation, leading to the development of shoots, roots, flowers, and the formation of a whole plant, typically categorized into indirect and direct organogenesis pathways [
28]. Among these receptors, zygotic embryos and somatic embryos emerge as the most favorable for inducing embryogenic callus tissue [
29]. Culturing both mature and immature embryos has been demonstrated to enhance regeneration and genetic transformation capabilities [
30]. The shoot apical meristem and leaves are common explants in trees [
31]. Furthermore, although the root system of trees can also serve as an explant, its regeneration capacity is comparatively lower than that of shoot apical meristems and leaves, necessitating additional optimization conditions [
32,
33]. Cotyledons are also present as suitable receptors for genetic transformation in several tree species, such as pine and eucalyptus. For example, the well-developed cotyledons of
Pinus nigra embryos were dissected into small pieces, and protoplasts were subsequently obtained through enzymatic digestion. Both the electroporation procedure and the particle bombardment procedure were employed to transform
uidA into protoplasts, successfully enhancing the transient expression of
uidA in
P. nigra [
34].
Somatic embryos are one of the most commonly used types of transformation receptors induced and cultured from mature tree tissues [
35]. Utilizing GV3101
Agrobacterium-mediated RNAi, silencing of
PaWOX8/9 was conducted in embryos of
Norway spruce, resulting in disrupted orientation of the cell division plane at the basal part, consequently leading to an aberrant morphology [
36]. Moreover, within spruce species, zygotic embryos serve as another highly efficient conduit for genetic transformation [
30]. Nonetheless, somatic embryos also pose some challenges, including instability during induction and culture processes, along with difficulties in obtaining high-quality embryos. Thus, the judicious selection of transformation receptors tailored to the specific traits of the target tree species and research objectives emerges as paramount in the realm of tree genetic transformation. The RAPID method capitalizes on plant regenerative capacities by injecting
A. tumefaciens into meristematic tissues, inducing efficient transfection in newly formed tissues [
37]. It outperforms traditional methods with increased transformation efficiency, a shorter duration, and the absence of tissue culture requirements. Consequently, this innovation overcomes limitations in achieving rapid plant transformation, showing promise for application in various plant species with active regeneration capabilities.
3. Advancements in Tree Genetic Transformation Process
The initiation of genetic transformation in forest trees dates back to 1988, with subsequent significant changes by the end of the 20th century [
28]. Despite these advancements, several challenges still exist that hinder the effective implementation of genetic transformation in forest trees. One prominent challenge lies in the limited regenerative capacity observed in many forest tree species. Additionally, the long growth cycle of forest trees further complicates the timeline required for achieving mature transgenic individuals [
4]. These factors contribute to the substantial temporal and financial investments associated with research in forest genetic transformation.
The cultivation of forest trees presents a range of challenges due to their varied tissue culture requirements, which necessitate the use of carefully optimized culture media compositions and cultivation conditions. Promising advancements in transformation technologies have led to significant progress, particularly when coupled with the development of forest embryos and the application of cutting-edge techniques [
9]. Several recent reviews have explored the new technology of genetic transformation of forest trees in great detail [
4,
8,
9]. Given the comprehensive coverage of these reviews, this section provides a concise overview focusing specifically on nanoparticle-mediated gene transformation, DNA-free gene editing technology and several novel delivery carriers.
3.1. Nanoparticle-Meditated Gene Transformation
With the continuous advancements in molecular biology and genetic engineering technologies, emerging gene delivery techniques have been introduced into tree genetic transformation. For example, methods like particle bombardment or electroporation enable the direct introduction of exogenous DNA into tree tissues, thereby improving the efficiency and stability of gene transformation [
38,
39]. In addition, a novel method utilizing nanoparticles (NPs) to facilitate gene transformation has emerged as a solution for the challenge posed by the plant cell wall [
40]. By utilizing this approach, DNA or RNA molecules can be precisely transferred into plants, resulting in either temporary or permanent genetic modifications [
41]. For instance, LDH-DNA bioconjugates as sandwich nanostructures can efficiently carry DNA into the nucleus in BY-2 suspension cells serve novel molecular delivery systems [
42]. In the future, with the target-specific delivery of NPs, the efficiency and success rate of genetic transformation in trees can be significantly improved.
3.2. Optimized Gene Editing Technologies
In pursuit of stable expression of exogenous genes and desired phenotypic changes, extensive work is conducted by researchers to optimize gene regulation. This includes selecting appropriate promoters and terminators, adjusting transgene copy numbers and insertion sites, and optimizing integration methods into the genome, ultimately improving exogenous gene expression and precise regulation of target genes in trees [
43]. For example, the use of RNA interference and gene editing technologies, such as the CRISPR-Cas9 system, further optimize the selection and improvement of tree genetic materials [
44]. These emerging technologies provide researchers with more possibilities for selecting and manipulating genetic materials. However, in woody species, except for poplar, the low transformation efficiency and in vitro regeneration capability, along with their inherently slow growth rate, pose significant bottlenecks for the more widespread implementation of genome editing technologies. In order to shorten the juvenile phase of woody plants and promote early flowering to ensure precocity, overexpression of the
BpMADS4 gene can be employed [
45]. Flachowsky et al. reported that overexpression of
BpMADS4 in apple significantly reduced the juvenile phase and achieved early flowering [
46]. Heterologous expression of FT from various donor species has been shown to shorten the generation time in
European plum, Eucalyptus,
Populus, and sweet orange [
47].
The uORF in eukaryotic mRNA regulates translation by inhibiting the main coding open reading frame. CRISPR-Cas9 editing of uORF in rice mutants with altered traits offers a universal approach for predictable gene expression fine-tuning in molecular design breeding [
48]. Artificial intelligence enhances large-scale protein structure prediction, with AI-assisted methods establishing a high-throughput clustering technique based on tertiary structures. This aids in exploring deaminase functional structures and identifying novel scaffold components [
49]. Optimized gene editing methods increasingly serve as effective tools for tree genetic transformation and mitigating non-biological stressors.
3.3. DNA Free Gene Editing Technology
Traditional stable transformation, although widely used for genome editing in plants, requires considerable time and labor to generate DNA-free gene-edited crops through genetic segregation [
50]. To improve efficiency, methods like fluorescent labeling and resistance screening are employed in CRISPR/Cas9 vectors [
51]. Using microscopy, non-fluorescent transgenic materials are excluded, or resistance-sensitive methods are used to eliminate materials containing exogenous genes. The transgene killer CRISPR (TKC) system facilitates the self-elimination of CRISPR components, reducing time and labor for obtaining DNA-free plants with desired genomic modifications [
52]. Through the gene gun method, CRISPR/Cas9 plasmid DNA (TECCDNA) or transcribed RNA (TECCRNA) is directly introduced into somatic cell embryos, where endogenous nucleases rapidly degrade the introduced DNA or RNA [
53]. In contrast, RNA virus-mediated CRISPR/Cas9 gene editing ensures virus-free transgenic progeny plants, as RNA viruses cannot infect embryos or seeds, and their replication avoids integration into the host plant chromosomes [
54]. This system offers an efficient and reliable tool for generating genetically modified forest trees with minimal foreign DNA content.
3.4. Peptide Aptamer Meditated Non-Genetically Modified for Protein Function Interference
Peptide aptamers are polypeptide chains composed of 8–20 amino acid residues that can specifically interact with target molecules. They can disrupt protein–protein interactions and deactivate the functionality of the target protein without altering the gene structure, degrading mRNA, or modifying the protein structure [
55]. Peptide aptamers have great potential applications in plant functional genomics as “suppressors” that bind to target proteins in plants and inhibit their functions. A 16-amino acid peptide aptamer library was screened with rice MAGO protein as bait, yielding the specific interacting aptamer, PAP [
56]. PAP preferentially forms a disruptive heterodimer, PAP-MAGO, competing with MAGO-Y14 heterodimer formation and leading to phenotypic similarities between PAP-overexpressing rice plants and
OsMAGO- and
OsY14-RNAi plants. Moreover, the employment of
Agrobacterium-mediated techniques for the delivery of viral vectors to plants, combined with the utilization of the amplification potential of RNA viruses, offers a promising avenue for the development of a “spray” technology capable of modifying crucial agronomic traits [
57].
Peptide aptamers have marked potential in forest tree research, offering valuable applications in targeted control of precise regulation of desirable traits related to growth, wood quality, and stress resistance. Designing peptide aptamers enables modulation of gene expression, allowing tailored approaches to enhance tree characteristics [
58]. Peptide aptamers can also enhance tree resilience to stress conditions such as drought and salinity by competitively inhibiting relevant molecular receptors, thereby effectively targeting and regulating tree responses. The peptide aptamer cPEP, specifically designed to target HSP101 in soybeans, exhibits a remarkable capability of enhancing their tolerance to heat stress [
59]. Notably, peptide aptamers provide the advantage of internal molecule regulation in plants without altering the genome or resorting to genetic editing or engineering methods. Consequently, peptide aptamers can be externally applied through techniques like spraying or irrigation, facilitating trait improvement in tree species with limited genetic transformation systems.
This entry is adapted from the peer-reviewed paper 10.3390/f15030441