Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Forestry
森林是一种重要的自然资源,在气候调节和维持生物多样性方面发挥着至关重要的作用。然而,森林树木的生长和发育越来越受到环境压力的增加,特别是有害的非生物压力源的挑战。为了应对这些挑战,基因转化技术已成为有效的解决方案。尽管林木遗传转化存在延长生命周期、遗传多样性、种间变异和复杂的再生系统等各种困难,但在树木基因编辑、转基因技术和外源分子递送方法方面取得了重大研究进展。这些技术有可能提高树木质量,提高生产力,并提高对非生物胁迫的抵抗力。
- genetic transformation
- transformation receptor
- abiotic stress
- nanoparticle
- peptide aptamer
1. 引言
森林是一种重要的自然资源,其产量和质量对人类生活质量和经济发展有直接影响[1,2]。传统的育种方法不足以应对不断升级的环境压力和病虫害带来的复杂挑战。此外,树木的遗传改良还受到寿命长、世代时间长、性成熟晚和遗传多样性有限等问题的制约[3,4]。因此,树木遗传转化成为深入研究的重点。这个过程涉及利用现代生物技术方法将外源基因或其他遗传物质引入植物细胞,从而改变其遗传特征。基因组编辑技术是修改、添加或删除基因组内基因的有效方法[5]。近年来,顺基因已成为一种不同于转基因的技术,因为它排除了从其他物种引入基因的可能性[6]。有时,基因编辑技术被用于将合成或人工工程基因引入宿主细胞[7]。这些进展推动了农业、林业和环境保护等领域的重大研究进展,显示出广阔的应用前景[8,9]。
树木遗传转化成为一种有效的方法,可增强抗逆性、提高产量和提高质量(图1)。例如,耐旱基因的引入使树木能够在干旱环境中茁壮成长。此外,改变树木的生长模式和木质素含量可以提高耐盐性[10]。
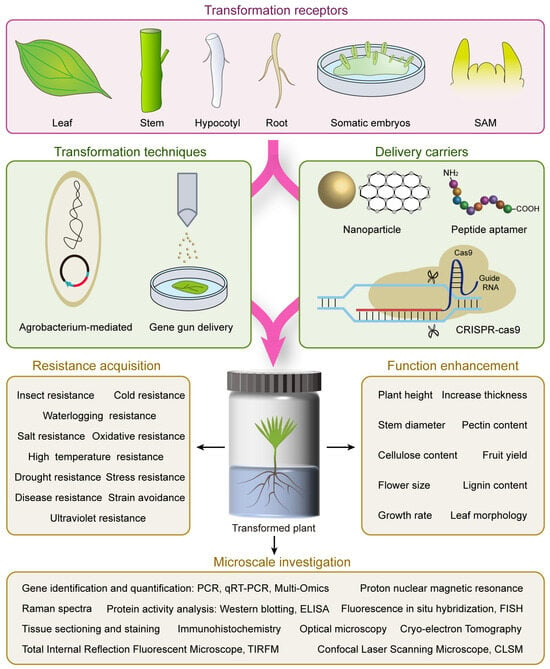
图 1.表意文字显示了森林树木遗传转化中常用的实验程序和转化受体以及后续研究。该过程主要由三个部分组成,包括转化受体的选择和制备,利用适当的转化技术将递送载体引入转化受体,最后对成功转化的植株进行抗逆性测试、功能分析和微观研究。
2. 树木遗传转化技术与转化受体
随着分子生物学和基因工程技术的发展,树木遗传转化技术有了显著的改进和完善。目前常用的树木遗传转化方法包括基因枪法和农杆菌介导法[11,12]。转化受体的选择是影响植物遗传转化的重要因素。转化受体是从可进行基因改造的植物组织中分离出来的,例如器官、细胞或原生质体。在受控的人工条件下,进行转化受体的培养以获得再生的转基因全株或实施生产具有经济价值的产品的技术[13]。由于建立树木再生系统的挑战,用于树木遗传转化的合适转化受体的选择有限[14]。通常,使用组织培养和再生技术在体外培养和再生树细胞或组织以获得转化受体[15]。
2.1. 常见的树木遗传转化技术
常见的遗传转化系统可分为两种类型:基因枪和农杆菌介导的方法。1994年,GUS通过基因枪成功转移到巴西橡胶的花药来源的愈伤组织中[16]。来自苏云金芽孢杆菌的合成 CRY1Ac 已被用于通过基因枪转化 Pinus taeda。这些转基因植物对点状石斛(Dendrolimus punctatus Walker)表现出高水平的抗性[17]。农杆菌介导的转化是树木遗传转化的首选方法。土壤中的农杆菌感染许多双子叶植物和裸子植物的受伤部位。它在浸润细胞时将 T-DNA 插入植物基因组,通过减数分裂确保稳定的遗传。这为农杆菌介导的植物遗传转化奠定了理论基础[18]。根癌农杆菌是植物遗传转化研究中使用的主要菌株之一。通过利用LBA4404、GV3101和EHA105等农杆菌菌株,在冷杉肾冷杉、桔梗和辐射松中成功产生了转基因植株[18,19,20]。根茎农杆菌是另一种常用的农杆菌类型。利用根茎芥进行树木遗传转化,有利于树木的基因功能研究和快速繁殖[21]。
选择合适的农杆菌菌株在树种的遗传转化中至关重要,因为它依赖于其独特的遗传性状、再生能力以及对不同农杆菌菌株的反应[22]。由根癌曲霉介导的叶盘转化由Horsch等[23]开发。利用叶盘可以实现杨叶的遗传转化,效率可达80%以上[24]。然而,根据我们之前的研究,这种方法在针叶树中实施具有挑战性。体细胞胚发生可用于落叶松(如马尾松(Pinus massoniana)和落叶松(Larix gmelinii)的遗传转化,但目前在油松(Pinus tabuliformis)中仍无法实现[25]。
2.2. Transformation Receptor Used in Forest Tree Genetic Transformation
Due to the intrinsic factors of trees and the difficulty in establishing regeneration systems, the transformation receptors suitable for tree genetic transformation primarily include mature and immature zygotic embryos, somatic embryos, seedlings, shoot meristems, and cotyledons [25,26,27]. Induced transformation receptors facilitate organ differentiation, leading to the development of shoots, roots, flowers, and the formation of a whole plant, typically categorized into indirect and direct organogenesis pathways [28]. Among these receptors, zygotic embryos and somatic embryos emerge as the most favorable for inducing embryogenic callus tissue [29]. Culturing both mature and immature embryos has been demonstrated to enhance regeneration and genetic transformation capabilities [30]. The shoot apical meristem and leaves are common explants in trees [31]. Furthermore, although the root system of trees can also serve as an explant, its regeneration capacity is comparatively lower than that of shoot apical meristems and leaves, necessitating additional optimization conditions [32,33]. Cotyledons are also present as suitable receptors for genetic transformation in several tree species, such as pine and eucalyptus. For example, the well-developed cotyledons of Pinus nigra embryos were dissected into small pieces, and protoplasts were subsequently obtained through enzymatic digestion. Both the electroporation procedure and the particle bombardment procedure were employed to transform uidA into protoplasts, successfully enhancing the transient expression of uidA in P. nigra [34].
Somatic embryos are one of the most commonly used types of transformation receptors induced and cultured from mature tree tissues [35]. Utilizing GV3101 Agrobacterium-mediated RNAi, silencing of PaWOX8/9 was conducted in embryos of Norway spruce, resulting in disrupted orientation of the cell division plane at the basal part, consequently leading to an aberrant morphology [36]. Moreover, within spruce species, zygotic embryos serve as another highly efficient conduit for genetic transformation [30]. Nonetheless, somatic embryos also pose some challenges, including instability during induction and culture processes, along with difficulties in obtaining high-quality embryos. Thus, the judicious selection of transformation receptors tailored to the specific traits of the target tree species and research objectives emerges as paramount in the realm of tree genetic transformation. The RAPID method capitalizes on plant regenerative capacities by injecting A. tumefaciens into meristematic tissues, inducing efficient transfection in newly formed tissues [37]. It outperforms traditional methods with increased transformation efficiency, a shorter duration, and the absence of tissue culture requirements. Consequently, this innovation overcomes limitations in achieving rapid plant transformation, showing promise for application in various plant species with active regeneration capabilities.
3. Advancements in Tree Genetic Transformation Process
The initiation of genetic transformation in forest trees dates back to 1988, with subsequent significant changes by the end of the 20th century [28]. Despite these advancements, several challenges still exist that hinder the effective implementation of genetic transformation in forest trees. One prominent challenge lies in the limited regenerative capacity observed in many forest tree species. Additionally, the long growth cycle of forest trees further complicates the timeline required for achieving mature transgenic individuals [4]. These factors contribute to the substantial temporal and financial investments associated with research in forest genetic transformation.
The cultivation of forest trees presents a range of challenges due to their varied tissue culture requirements, which necessitate the use of carefully optimized culture media compositions and cultivation conditions. Promising advancements in transformation technologies have led to significant progress, particularly when coupled with the development of forest embryos and the application of cutting-edge techniques [9]. Several recent reviews have explored the new technology of genetic transformation of forest trees in great detail [4,8,9]. Given the comprehensive coverage of these reviews, this section provides a concise overview focusing specifically on nanoparticle-mediated gene transformation, DNA-free gene editing technology and several novel delivery carriers.
3.1. Nanoparticle-Meditated Gene Transformation
With the continuous advancements in molecular biology and genetic engineering technologies, emerging gene delivery techniques have been introduced into tree genetic transformation. For example, methods like particle bombardment or electroporation enable the direct introduction of exogenous DNA into tree tissues, thereby improving the efficiency and stability of gene transformation [38,39]. In addition, a novel method utilizing nanoparticles (NPs) to facilitate gene transformation has emerged as a solution for the challenge posed by the plant cell wall [40]. By utilizing this approach, DNA or RNA molecules can be precisely transferred into plants, resulting in either temporary or permanent genetic modifications [41]. For instance, LDH-DNA bioconjugates as sandwich nanostructures can efficiently carry DNA into the nucleus in BY-2 suspension cells serve novel molecular delivery systems [42]. In the future, with the target-specific delivery of NPs, the efficiency and success rate of genetic transformation in trees can be significantly improved.
3.2. Optimized Gene Editing Technologies
In pursuit of stable expression of exogenous genes and desired phenotypic changes, extensive work is conducted by researchers to optimize gene regulation. This includes selecting appropriate promoters and terminators, adjusting transgene copy numbers and insertion sites, and optimizing integration methods into the genome, ultimately improving exogenous gene expression and precise regulation of target genes in trees [43]. For example, the use of RNA interference and gene editing technologies, such as the CRISPR-Cas9 system, further optimize the selection and improvement of tree genetic materials [44]. These emerging technologies provide researchers with more possibilities for selecting and manipulating genetic materials. However, in woody species, except for poplar, the low transformation efficiency and in vitro regeneration capability, along with their inherently slow growth rate, pose significant bottlenecks for the more widespread implementation of genome editing technologies. In order to shorten the juvenile phase of woody plants and promote early flowering to ensure precocity, overexpression of the BpMADS4 gene can be employed [45]. Flachowsky et al. reported that overexpression of BpMADS4 in apple significantly reduced the juvenile phase and achieved early flowering [46]. Heterologous expression of FT from various donor species has been shown to shorten the generation time in European plum, Eucalyptus, Populus, and sweet orange [47].
The uORF in eukaryotic mRNA regulates translation by inhibiting the main coding open reading frame. CRISPR-Cas9 editing of uORF in rice mutants with altered traits offers a universal approach for predictable gene expression fine-tuning in molecular design breeding [48]. Artificial intelligence enhances large-scale protein structure prediction, with AI-assisted methods establishing a high-throughput clustering technique based on tertiary structures. This aids in exploring deaminase functional structures and identifying novel scaffold components [49]. Optimized gene editing methods increasingly serve as effective tools for tree genetic transformation and mitigating non-biological stressors.
3.3. DNA Free Gene Editing Technology
Traditional stable transformation, although widely used for genome editing in plants, requires considerable time and labor to generate DNA-free gene-edited crops through genetic segregation [50]. To improve efficiency, methods like fluorescent labeling and resistance screening are employed in CRISPR/Cas9 vectors [51]. Using microscopy, non-fluorescent transgenic materials are excluded, or resistance-sensitive methods are used to eliminate materials containing exogenous genes. The transgene killer CRISPR (TKC) system facilitates the self-elimination of CRISPR components, reducing time and labor for obtaining DNA-free plants with desired genomic modifications [52]. Through the gene gun method, CRISPR/Cas9 plasmid DNA (TECCDNA) or transcribed RNA (TECCRNA) is directly introduced into somatic cell embryos, where endogenous nucleases rapidly degrade the introduced DNA or RNA [53]. In contrast, RNA virus-mediated CRISPR/Cas9 gene editing ensures virus-free transgenic progeny plants, as RNA viruses cannot infect embryos or seeds, and their replication avoids integration into the host plant chromosomes [54]. This system offers an efficient and reliable tool for generating genetically modified forest trees with minimal foreign DNA content.
3.4. Peptide Aptamer Meditated Non-Genetically Modified for Protein Function Interference
Peptide aptamers are polypeptide chains composed of 8–20 amino acid residues that can specifically interact with target molecules. They can disrupt protein–protein interactions and deactivate the functionality of the target protein without altering the gene structure, degrading mRNA, or modifying the protein structure [55]. Peptide aptamers have great potential applications in plant functional genomics as “suppressors” that bind to target proteins in plants and inhibit their functions. A 16-amino acid peptide aptamer library was screened with rice MAGO protein as bait, yielding the specific interacting aptamer, PAP [56]. PAP preferentially forms a disruptive heterodimer, PAP-MAGO, competing with MAGO-Y14 heterodimer formation and leading to phenotypic similarities between PAP-overexpressing rice plants and OsMAGO- and OsY14-RNAi plants. Moreover, the employment of Agrobacterium-mediated techniques for the delivery of viral vectors to plants, combined with the utilization of the amplification potential of RNA viruses, offers a promising avenue for the development of a “spray” technology capable of modifying crucial agronomic traits [57].
Peptide aptamers have marked potential in forest tree research, offering valuable applications in targeted control of precise regulation of desirable traits related to growth, wood quality, and stress resistance. Designing peptide aptamers enables modulation of gene expression, allowing tailored approaches to enhance tree characteristics [58]. Peptide aptamers can also enhance tree resilience to stress conditions such as drought and salinity by competitively inhibiting relevant molecular receptors, thereby effectively targeting and regulating tree responses. The peptide aptamer cPEP, specifically designed to target HSP101 in soybeans, exhibits a remarkable capability of enhancing their tolerance to heat stress [59]. Notably, peptide aptamers provide the advantage of internal molecule regulation in plants without altering the genome or resorting to genetic editing or engineering methods. Consequently, peptide aptamers can be externally applied through techniques like spraying or irrigation, facilitating trait improvement in tree species with limited genetic transformation systems.
This entry is adapted from the peer-reviewed paper 10.3390/f15030441
This entry is offline, you can click here to edit this entry!
