Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The progress of artificial intelligence algorithms in digital image processing and automatic diagnosis studies of the eye disease glaucoma has been growing and presenting essential advances to guarantee better clinical care for the population.
- deep learning
- glaucoma
- image analysis
- artificial intelligence
1. Introduction
Glaucoma is a multifactorial neuropathy that can affect the fundus of the eye, causing gradual loss of vision and, in severe cases, blindness. Traditionally, the diagnosis of glaucoma is applied with the help of readily available ophthalmological teams and highly specialized equipment. The sensitivity of the diagnosis is generally high, as tests applied in ophthalmology offices have the clinical potential to identify virtually all cases of the disease. However, despite this sophisticated diagnostic scenario, the silent and slow evolution of the disease, the costs of exams and consultations, and the lack of access to public ophthalmological services in many cases prevent thousands of people from consulting an ophthalmologist during the early stages of this neuropathy. This contributes to the fact that around 70% of the patients are self-diagnosed, that is, alerted by their own visual impairment and not by an appropriate early diagnosis [1][2].
Glaucoma is considered a global problem; even in developed countries, it is estimated that at least 50% of patients with glaucoma do not know of their condition. This percentage is even worse in low-income countries [3]. It is considered a progressive, chronic, and incurable pathology; however, it can generally be efficiently controlled when treatment begins in the early stages of the disease.
There are several types of glaucoma: open-angle glaucoma, angle-closure glaucoma, congenital glaucoma and secondary glaucoma [4][5]. However, they all cause damage to the optic nerve, which in most cases occurs slowly, initially leading to the loss of midperipheral vision. In advanced stages, it affects central vision, leading to irreversible blindness.
Damage to the optic nerve can be analyzed using fundus examinations, also known as ophthalmoscopy or fundoscopy. The ophthalmoscopy examination is performed on the back part of the eye (fundus), which includes the retina, optic disc, choroid, and blood vessels. The funduscopic examination can be performed with a variety of equipment, such as direct ophthalmoscopy, indirect ophthalmoscopy, and slit lamp ophthalmoscopy. Found in almost all ophthalmology offices, these devices offer ophthalmologists a detailed view of the eyeball. As shown in Figure 1, the brightest part of the retina represents the optic disc (OD), which contains an excavation known as the optical cup (OC), depicted by the whitest part of the interior of the optic disc. Therefore, if the size of the optic cup increases, it is considered one of the main indicators of glaucoma [2][6][7][8].
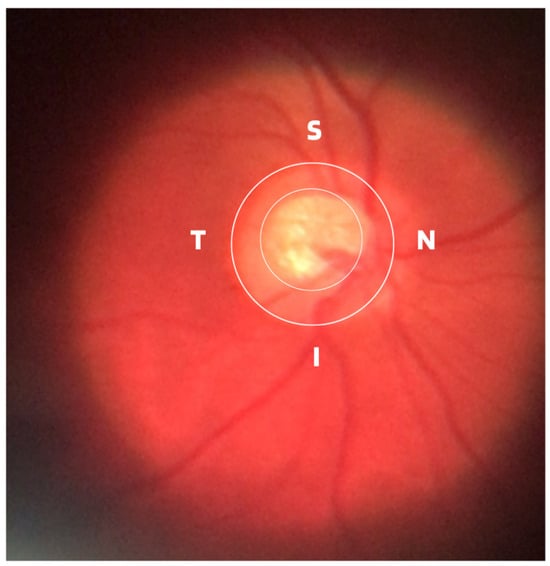
Figure 1. ISNT (Inferior (I), Superior (S), Nasal (N) and Temporal (T)) Rule.
In terms of the basic and traditional methods of diagnosing glaucoma, in addition to the fundus examination to examine the optic disc and the retinal nerve fiber layer (RNFL), ophthalmologists generally use tonometry and visual field tests as adjuncts. Tonometry is an exam to assess the degree of dysfunction and measures intraocular pressure (IOP) in millimeters of mercury (mmHg). The common eye pressure range is 10 to 21 mmHg, which is based on the average eye pressure level of a normal person. Although tonometry examination is very important in the management and treatment of glaucoma, it cannot be considered a diagnosis due to the presence of cases of normal pressure glaucoma [9]. Perimetry through the perimetry or campimetry exam, as is also known, the degree of functional impairment resulting from the disease is examined through the results of the obtained visual field map. In clinical practice, visual field testing identifies so-called blind spots (scotomas) and their locations in human vision and is therefore widely used as the gold standard to assess whether a patient suffers from typical functional glaucomatous damage [10].
Although the demographic and clinical characteristics associated with glaucoma are relatively well known, there is still no uniform definition of the diagnosis of this disease by ophthalmologists. In this way, many international efforts have been made to develop such a definition, but no real consensus standard has been reached. Therefore, those with an IOP greater than 21 mmHg, accompanied by characteristic damage to the optic disc or defects in the visual field compatible with glaucoma, are generally included as glaucomatous [11]. Due to this particularity, it is important to assess and document the appearance of an increase in the cup-to-disc ratio as a way of evaluating possible structural damage caused by the disease, as well as accompanying the patient to treatment or routine appointments. Therefore, from ophthalmoscopy images, ophthalmologists can evaluate at least four important informative characteristics of glaucoma, such as cup/disc ratio, inferior (I), superior (S), nasal (N), and temporal (T) rule (ISNT), cup asymmetry, and in addition other structural damage caused to the optic disc, namely the following:
-
Cup-to-Disc Ratio (CDR): An abnormal increase in disc cupping is important in the diagnosis of glaucoma; however, many people may have increased nerve cupping and not necessarily have glaucoma. This is especially true for myopic people, who tend to have a larger optical disc and consequently a larger optical cup. Therefore, during the diagnosis of glaucoma, it is important to assess not only the optical cup but also the cup-to-disc ratio (CDR). For better understanding, the CDR measurement is calculated from the relationship between the vertical diameter of the excavation (VCD) and the vertical diameter of the disc (VDD), as shown in Figure 2.To calculate the CDR ratio, the optical disc must be divided into 10 equal parts, as in Figure 3, and then the excavation scope must be taken into account in each division made. Therefore, it is considered a fractional percentage measurement, generally made horizontally, and can vary greatly between normal individuals. However, optical excavations greater than 0.65 indicate possible abnormalities, suggesting further investigation [2][12].
-
ISNT Rule: The border formed between the optic cup and the optic disc, called the neuroretinal ring or neural ring, is also considered an indication of glaucoma, for which there is a rule called ISNT, which alludes to the orientation (inferior, superior, nasal, and temporal) of the edges in the image of the fundus, as shown in Figure 1. When considering the ISNT rule, in nonglaucomatous eyes, it is suggested that the thickness of the neural ring should be greatest in the inferior quadrant, followed by the superior, nasal, and temporal quadrants. Misalignment in the guidelines of this rule leads to suspicion of glaucoma [13].
-
Cup-to-disc ratio (CDR) asymmetry: The CDR relationship between both eyes is symmetric in most people, and asymmetry is an important sign of suspected glaucomatous damage. This is due to the observation that 1% to 6% normal adults may have a discrepancy of 0.2 in the cup/disc ratio, while 1% of the general population may have an asymmetry of 0.3. Therefore, cup asymmetry is a finding on ophthalmological examination that requires additional tests to rule out the presence of glaucoma or other possible complications [14][15].
-
Other structural damage to the optic disc: The main descriptions of these types of damage related to glaucoma are as follows [2][16][17]:
-
Changes in RNFL: the presence of defects located in the retinal nerve fiber layer is called Hoyt’s sign and is characterized by a dark area that extends and widens from the optic disc, exhibiting an arched shape.
-
Peripapillary atrophy: According to the ophthalmological appearance, peripapillary atrophy can be divided into a peripheral alpha zone and a central beta zone. The alpha zone is characterized by patchy hypopigmentation and thinning of the layers of the chorioretinal tissue. It is laterally adjacent to the retina and medially in contact with the beta area, with the sclera and large choroidal vessels visible. In normal eyes, the alpha and beta areas are usually located in the temporal area, followed by the inferior and superior areas. In glaucomatous eyes, the beta area is more present in the temporal region and its extension is associated with thinning of the RNFL.
-
Excavation of the optic disc: In addition to disc excavation, the neuroretinal ring or neural rim must also be observed, as excavation is influenced by the size of the optic disc.
-
Disc hemorrhage: The presence of peripapillary hemorrhages is an important sign in both the diagnosis and the monitoring of glaucoma. Therefore, vessel deflection and nasal excavation must be examined.
-
Denudation of the lamina, cribriform: the presence of visible extinction of the cribriform lamina to the edge of the optic disc is called a notch, which represents the evolution of a defect located in the neural rim until there is a complete absence of tissue in the region, which exposes the cribriform lamina and allows visualization of its pores. Although it is very suggestive of glaucoma, this sign is not characteristic of the disease.
-
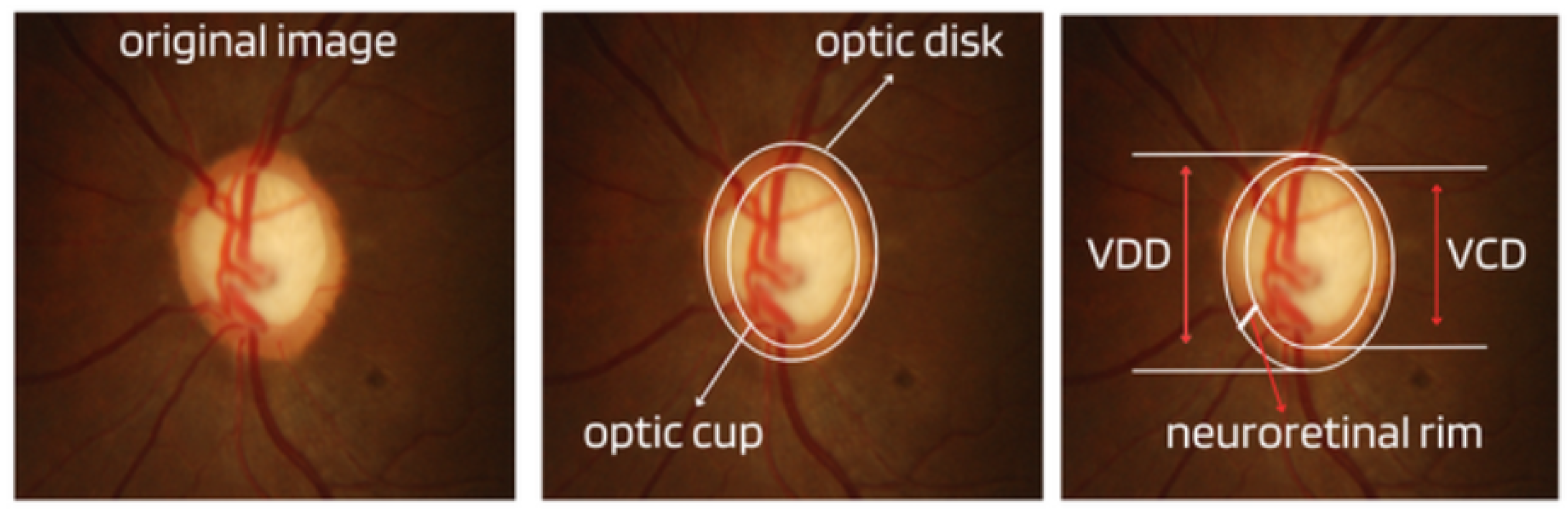
Figure 2. Measures considered in the CDR calculation.
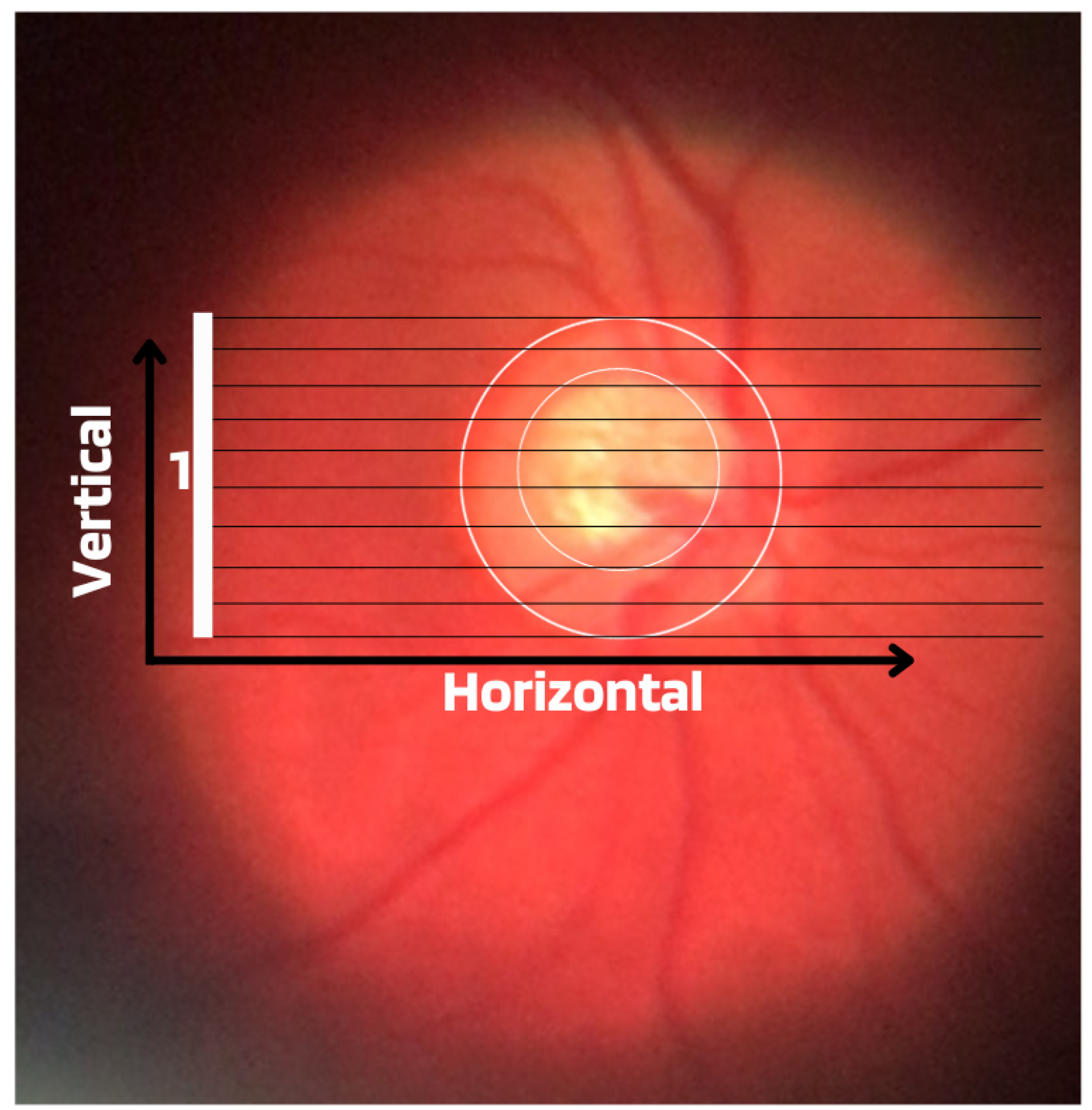
Figure 3. Example of CDR calculation with figure showing excavation of 0.6.
Regarding the difficulties associated with the diagnosis of glaucoma, it is considered that in cases in the moderate or advanced stages of the disease, the diagnosis is usually more simplified. However, the best way is to detect early glaucoma, which is essential for adequate treatment, mainly because quality of life can be altered even with slight loss of visual field [18]. However, the early identification of this disease, although important, can be challenging for several reasons, including glaucomatous characteristics that can be ambiguous in the optic disc region, RNFL, or visual field results at the beginning of the disease.
2. Epidemiology
According to the World Health Organization (WHO), at least 2.2 billion people around the world suffer from some type of visual impairment. In almost half of the cases, this deficiency could have been avoided or has not yet been treated. When considering these data, it is inferred that today millions of people live with visual impairment or blindness that could have been avoided but unfortunately were not.
Although the exact number is unknown, it is estimated that 11.9 million people worldwide have moderate or severe visual impairment or blindness due to eye diseases such as glaucoma, trachoma (an inflammatory condition that affects the conjunctiva and cornea), and diabetic retinopathy, a chronic complication of diabetes mellitus [19][20][21].
Visual impairment and blindness can have a major impact on the daily lives of people affected by such disabilities, since vision is the dominant sense for humans at all stages of life. However, research estimates that by 2030, around 95.4 million people worldwide will have glaucoma.
Visual impairment, in addition to being detrimental to patient quality of life, also presents a huge global financial burden, as demonstrated by previous research that estimated the costs of lost productivity. These costs can be divided into direct costs and indirect costs. Direct costs include medications, surgeries, medical consultations, hospitalizations, and complementary examinations. Indirect medical costs include mainly the economic impacts caused by visual impairment on work productivity.
Although glaucoma generally progresses slowly and is underdiagnosed worldwide, it is the most common cause of irreversible blindness globally, yet it can be prevented. The disease is considered preventable because, if detected early, there are ways to control it, but global statistics show that due to underdiagnosis, the result is a large number of blind people. This problem can be even more serious in low-income or underdeveloped countries, such as Brazil, considered by the World Inequality Lab report in 2018 [22] as one of the countries with the highest social and income inequality in the world, marked by extreme levels for many consecutive years.
Although statistical numbers of underdiagnosis in the general population combined with the need for early diagnosis to prevent blindness may suggest that glaucoma is a good candidate for population screening, studies have shown that, at least in countries such as the United Kingdom and Finland, the detection of population-based glaucoma using traditional diagnostic methods is not feasible due to the high cost of implementation and maintenance and the relatively low prevalence of the disease in the general population, which is approximately 3.5% [23][24]. Similarly, the US Preventive Services Task Force [25], with the support of the American Academy of Family Physicians [26], does not recommend screening for glaucoma in the primary care setting, citing insufficient evidence to assess its implications, benefits, or harms.
3. Scientific and Technological Advances in Artificial Intelligence
In recent years, scientific and technological advances have opened up a wide range of clinical and research opportunities in the field of ophthalmological care, which can help combat glaucoma. In this way, artificial intelligence technologies have proven effective in areas of medicine such as radiology, pathology, dermatology, etc. All of these studies are in related areas that share parallels with ophthalmology because of their deep roots in diagnostic imaging.
The term artificial intelligence is a technology that covers several areas of knowledge and generally refers to the development of computational systems capable of performing tasks that mimic human intelligence. More recently, through machine learning and algorithms known as artificial neural networks (ANN) and deep neural networks (DNN) many advances have been possible [27][28].
The concept of machine learning encompasses a variety of methodologies, such as random forests [29], K-nearest neighbors (KNN) [30], support vector machines (SVM) [31], naive bayes [32], and artificial neural networks [27]. All of these technologies are aimed at pattern recognition, statistical regression, and data classification processes. Among machine learning algorithms, deep learning technology stands out, which has been at the forefront of the development and advances in computing and big data in recent years, mainly with the introduction and development of convolutional neural network (CNN) networks, proposed by researcher Yann LeCun [33] and especially used in the areas of pattern recognition and digital image classification.
The networks presented are algorithms that require a lot of data for training, but often there are not enough data, especially when considering clinical information. Therefore, a widely used technique that allows neural networks to be applied to small data sets is the process of transfer learning, considered the method of transferring knowledge acquired during training in a certain domain (a database) to be applied in another domain, that is, another similar problem. In view of this, algorithms that offer this technology are called pre-trained. One of the conveniences of using pre-trained networks is that they already have defined weights; that is, the weights are initialized with values obtained from already completed training.
Still in transfer learning, the ImageNet Large Scale Visual Recognition Challenge (ILSVRC) is an annual competition run by the ImageNet team since 2010, in which research teams evaluate the performance of computer vision and machine learning algorithms on various transfer learning tasks. visual recognition, such as object classification and localization [34]. ImageNet is a project aiming to provide large libraries of images for use in pre-training algorithms to be used in various other tasks and has been fundamental for advancing research in computer vision and deep learning. This database contains more than 14 million images, divided into more than 20,000 categories.
Due to data deficiency and other purposes, generative adversarial networks (GANs) also emerged, a machine learning architecture that consists of two networks that ’fight’ against each other (damage to the environment). The potential of GANs is enormous because they can learn to imitate any data distribution in the following way: First, a neural network called a generator generates new data instances, while another neural network called a discriminator evaluates their authenticity. In this way, the generator produces false images in the hope that the false images will even be considered real by the discriminator. With this exchange of information, the generator learns to generate plausible data, while the discriminator learns to distinguish false data from the generator. The discriminator penalizes the generator for producing concrete results, and with this, the generator improves more and more.
Training of GANs networks is carried out using real data instances as positive and fake data instances created by the generator as negative. After training, the classifier classifies the real and fake generator data and propagates the discriminator loss through the discriminator network to update the weights [35].
All these artificial intelligence technologies, regardless of the difficulty in finding large sets of public data or the algorithmic model used, show the great commitment of researchers to spread scientific growth seeking to find valid and effective solutions in the diagnosis of glaucoma. In this way, with respect to the application of artificial intelligence to ophthalmology, in addition to studies aimed at the automatic diagnosis of glaucoma, this technology also focuses on studies on the diagnosis of diseases such as cataracts, age-related macular degeneration, diabetic retinopathy, and others, showing that there is a set of ophthalmological diseases that can receive greater attention considering the use of deep learning.
Regarding the ophthalmological scenario of glaucoma, the use of artificial intelligence appears as an auxiliary tool in the diagnosis of the disease by detecting changes present in the OCT results, the results of the visual field exam, and mainly in the images of the fundus. This is because, despite the potential to apply automation to different types of ophthalmic images, fundus images (i.e., images obtained with conventional ophthalmic equipment) have gained prominence in many related works due to the availability, quality, and cost effectiveness of acquisition.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics14050530
References
- Tan, N.Y.; Friedman, D.S.; Stalmans, I.; Ahmed, I.I.K.; Sng, C.C. Current opinion in ophthalmology. Curr. Opin. Ophthalmol. 2020, 31, 91–100.
- Bragança, C.P.; Torres, J.M.; De Almeida Soares, C.P. Inteligência artificial e diagnóstico do glaucoma. Braz. Appl. Sci. Rev. 2023, 7, 683–707.
- Heijl, A.; Bengtsson, B.; Oskarsdottir, S.E. Prevalence and severity of undetected manifest glaucoma: Results from the early manifest glaucoma trial screening. Ophthalmology 2013, 120, 1541–1545.
- Salmon, J.F. Clinical Ophthalmology: A Systematic Approach, 10th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2024.
- NIH National Library of Medicine. Medical Encyclopedia . Medical Encyclopedia: Glaucoma. 2023. Available online: https://medlineplus.gov/ency/article/001620.htm (accessed on 20 February 2024).
- Giorgis, A.T.; Alemu, A.M.; Arora, S.; Gessesse, G.W.; Melka, F.; Woldeyes, A.; Amin, S.; Kassam, F.; Kurji, A.K.; Damji, K.F. Results from the first teleglaucoma pilot project in Addis Ababa, Ethiopia. J. Glaucoma 2019, 28, 701–707.
- Smith, A.M.; Czyz, C.N. Neuroanatomy, cranial nerve 2 (Optic). In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Sociedade Brasileira de Glaucoma (SBC). Manual De Exame Em Glaucoma. 2015. Available online: https://www.sbglaucoma.org.br/medico/wp-content/uploads/2016/05/folder.pdf (accessed on 20 February 2024).
- Oshika, T.; Yoshitomi, F.; Oki, K. The pachymeter guide: A new device to facilitate accurate corneal thickness measurement. Jpn. J. Ophthalmol. 1997, 41, 426–427.
- Li, F.; Wang, Z.; Qu, G.; Song, D.; Yuan, Y.; Xu, Y.; Gao, K.; Luo, G.; Xiao, Z.; Lam, D.S.; et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med. Imaging 2018, 18, 1–7.
- Garway-Heath, D.F. Early diagnosis in glaucoma. Prog. Brain Res. 2008, 173, 47–57.
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The diagnosis and treatment of glaucoma. Dtsch. äRzteblatt Int. 2020, 117, 225.
- Khalil, T.; Usman Akram, M.; Khalid, S.; Jameel, A. Improved automated detection of glaucoma from fundus image using hybrid structural and textural features. IET Image Process. 2017, 11, 693–700.
- Arvind, H.; George, R.; Raju, P.; Ve, R.S.; Mani, B.; Kannan, P.; Vijaya, L. Optic Disc Dimensions and Cup-Disc Ratios among Healthy South Indians: The Chennai Glaucoma Study. Ophthalmic Epidemiol. 2011, 18, 189–197.
- Qiu, M.; Boland, M.V.; Ramulu, P.Y. Cup-to-Disc Ratio Asymmetry in U.S. Adults: Prevalence and Association with Glaucoma in the 2005–2008 National Health and Nutrition Examination Survey. Ophthalmology 2017, 124, 1229–1236.
- Tinku, R.S.J.; Diniz Filho, A. Simplificando o Diagnóstico e Tratamento do Glaucoma; Cultura Médica: Rio de Janeiro, Brazil, 2019.
- Jung, K.I.; Jeon, S.; Park, C.K. Lamina Cribrosa Depth is Associated With the Cup-to-Disc Ratio in Eyes With Large Optic Disc Cupping and Cup-to-Disc Ratio Asymmetry. J. Glaucoma 2016, 25, e536–e545.
- Tatham, A.J.; Weinreb, R.N.; Medeiros, F.A. Strategies for improving early detection of glaucoma: The combined structure–function index. Clin. Ophthalmol. 2014, 8, 611–621.
- World Health Organization. World Report on Vision. 2019. Available online: https://www.who.int/docs/default-source/documents/publications/world-vision-report-accessible.pdf (accessed on 20 February 2024).
- da Silva Negreiros, E.C.M.; dos Santos Silva, L.C.; de Araújo, A.C.R.; Dias, L.R.C.; de Moura, L.V.M.; Santa Rosa, I.M.; de Menezes Filho, J.M.; Marques, C.P.C. Mortalidade por Diabetes Mellitus no nordeste do Brasil no período de 2014 a 2018. Braz. J. Health Rev. 2023, 6, 14138–14155.
- Reis, T.M.; de Moraes Ramos, Y.T.; da Silva, Y.R.M.; Silva, R.A.; de Araújo, M.R.A.; de Araújo, W.M.; Beserra, I.Â.; da Cunha, A.D.R.; Silva, M.B.A. Análise de um triênio dos casos de tracoma em escolares residentes do município de Moreno. Braz. J. Health Rev. 2019, 2, 2273–2286.
- Alvaredo, F.; Chancel, L.; Piketty, T.; Saez, E.; Zucman, G. World Inequality Report 2018; Belknap Press: Cambridge, MA, USA, 2018.
- Vaahtoranta-Lehtonen, H.; Tuulonen, A.; Aronen, P.; Sintonen, H.; Suoranta, L.; Kovanen, N.; Linna, M.; Läärä, E.; Malmivaara, A. Cost effectiveness and cost utility of an organized screening programme for glaucoma. Acta Ophthalmol. Scand. 2007, 85, 508–518.
- Zaleska-Żmijewska, A.; Szaflik, J.P.; Borowiecki, P.; Pohnke, K.; Romaniuk, U.; Szopa, I.; Pniewski, J.; Szaflik, J. A new platform designed for glaucoma screening: Identifying the risk of glaucomatous optic neuropathy using fundus photography with deep learning architecture together with intraocular pressure measurements. Klin. Oczna/Acta Ophthalmol. Pol. 2020, 122, 1–6.
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Epling, J.W.; Jaén, C.R.; et al. Screening for primary open-angle glaucoma: US Preventive Services Task Force recommendation statement. JAMA 2022, 327, 1992–1997.
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. Primary open-angle glaucoma preferred practice pattern®. Ophthalmology 2021, 128, P71–P150.
- McCarthy, J.; Minsky, M.L.; Rochester, N.; Shannon, C.E. A proposal for the dartmouth summer research project on artificial intelligence, August 31, 1955. AI Mag. 2006, 27, 12.
- Russell, S.; Norvig, P. Artificial Intelligence: A Modern Approach, 4th ed.; Pearson: London, UK, 2020.
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32.
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27.
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297.
- Saritas, M.M.; Yasar, A. Performance analysis of ANN and Naive Bayes classification algorithm for data classification. Int. J. Intell. Syst. Appl. Eng. 2019, 7, 88–91.
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324.
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. Imagenet large scale visual recognition challenge. Int. J. Comput. Vis. 2015, 115, 211–252.
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial networks. Commun. ACM 2020, 63, 139–144.
This entry is offline, you can click here to edit this entry!
