Neurotrophic tyrosine receptor kinase (NTRK) has been a remarkable therapeutic target for treating different malignancies, playing an essential role in oncogenic signaling pathways. Groundbreaking trials like NAVIGATE led to the approval of NTRK inhibitors by the Food and Drug Administration (FDA) to treat different malignancies, significantly impacting current oncology treatment. Accurate detection of NTRK gene fusion becomes very important for possible targeted therapy. Various methods to detect NTRK gene fusion have been applied widely based on sensitivity, specificity, and accessibility.
- NTRK gene fusion
- NTRK inhibitor
- malignancies
1. Introduction
2. Different Methods for NTRK Gene Fusion Detection
3. TRK Biology and Ontogenesis
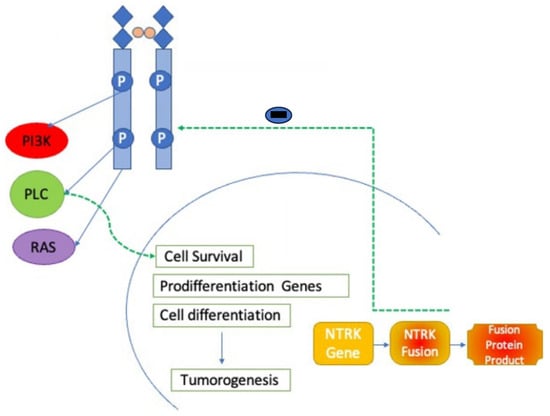
4. Mechanisms of Action
5. First-Generation NTRK Inhibitors
5.1. Larotrectinib
5.2. Entrectinib
6. Second-Generation NTRK Inhibitors
6.1. Repotrectinib
6.2. Selitrectinib
6.3. Taletrectinib
6.4. Other Agents
6.5. Mechanisms of Resistance to NTRK Inhibitors
6.6. Overall Side Effects and Effects of TRK Inhibition
7. Role of NTRK Inhibitors in Various Types of Cancers and Adverse Effects
7.1. NTRK Gene Fusion in Lung Cancer
7.2. NTRK Gene Fusion in Colorectal Cancer
7.3. NTRK Gene Fusion in Central Nervous System Malignancies
7.4. NTRK Gene Fusion in Thyroid Cancers
7.5. NTRK Gene Fusion in Hematological Malignancies
7.6. NTRK Gene Fusion in Sarcomas
7.7. NTRK Gene Fusion in Melanocytic Tumors
7.8. NTRK Gene Fusion in Salivary Gland Tumors
This entry is adapted from the peer-reviewed paper 10.3390/ijms25042366
References
- National Cancer Institute. FDA Approves Entrectinib for NTRK Fusion Cancers. Cancer Currents Blog. 16 August 2019. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2019/fda-entrectinib-ntrk-fusion (accessed on 1 January 2020).
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409.
- Lannon, C.L.; Sorensen, P.H. ETV6-NTRK3: A chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin. Cancer Biol. 2005, 15, 215–223.
- Pulciani, S.; Santos, E.; Lauver, A.V.; Long, L.K.; Aaronson, S.A.; Barbacid, M. Oncogenes in solid human tumours. Nature 1982, 300, 539–542.
- Ruiz-Cordero, R.; Ng, D.L. Neurotrophic receptor tyrosine kinase (NTRK) fusions and their role in cancer. Cancer Cytopathol. 2020, 128, 775–779.
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739.
- Marcus, L.; Donoghue, M.; Aungst, S.; Myers, C.E.; Helms, W.S.; Shen, G.; Zhao, H.; Stephens, O.; Keegan, P.; Pazdur, R. FDA Approval Summary: Entrectinib for the Treatment of NTRK gene Fusion Solid Tumors. Clin. Cancer Res. 2021, 27, 928–932.
- Connor, A.; Perez-Ordoñez, B.; Shago, M.; Skálová, A.; Weinreb, I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: Expanded morphologic and immunohistochemical spectrum of a recently described entity. Am. J. Surg. Pathol. 2012, 36, 27–34.
- Skálová, A.; Vanecek, T.; Simpson, R.H.; Laco, J.; Majewska, H.; Baneckova, M.; Steiner, P.; Michal, M. Mammary Analogue Secretory Carcinoma of Salivary Glands: Molecular Analysis of 25 ETV6 Gene Rearranged Tumors with Lack of Detection of Classical ETV6-NTRK3 Fusion Transcript by Standard RT-PCR: Report of 4 Cases Harboring ETV6-X Gene Fusion. Am. J. Surg. Pathol. 2016, 40, 3–13.
- Wong, D.; Yip, S.; Sorensen, P.H. Methods for Identifying Patients with Tropomyosin Receptor Kinase (TRK) Fusion Cancer. Pathol. Oncol. Res. 2020, 26, 1385–1399.
- Martin-Zanca, D.; Oskam, R.; Mitra, G.; Copeland, T.; Barbacid, M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol. Cell Biol. 1989, 9, 24–33.
- Klein, R.; Jing, S.Q.; Nanduri, V.; O’Rourke, E.; Barbacid, M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 1991, 65, 189–197.
- Soppet, D.; Escandon, E.; Maragos, J.; Middlemas, D.S.; Reid, S.W.; Blair, J.; Burton, L.E.; Stanton, B.R.; Kaplan, D.R.; Hunter, T.; et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991, 65, 895–903.
- Frade, J.M.; Barde, Y.A. Nerve growth factor: Two receptors, multiple functions. BioEssays: News and reviews in molecular, cellular and developmental biology. Bioessays 1998, 20, 137–145.
- Patel, T.D.; Jackman, A.; Rice, F.L.; Kucera, J.; Snider, W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 2000, 25, 345–357, Erratum in Neuron 2003, 37, 183.
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5455–5463.
- Rajagopal, R.; Chen, Z.Y.; Lee, F.S.; Chao, M.V. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J. Neurosci. 2004, 24, 6650–6658.
- Geiger, T.R.; Song, J.Y.; Rosado, A.; Peeper, D.S. Functional characterization of human cancer-derived TRKB mutations. PLoS ONE 2011, 6, e16871.
- Harada, T.; Yatabe, Y.; Takeshita, M.; Koga, T.; Yano, T.; Wang, Y.; Giaccone, G. Role and relevance of TrkB mutations and expression in non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2638–2645.
- Miranda, C.; Mazzoni, M.; Sensi, M.; Pierotti, M.A.; Greco, A. Functional characterization of NTRK1 mutations identified in melanoma. Genes Chromosomes Cancer 2014, 53, 875–880.
- Tomasson, M.H.; Xiang, Z.; Walgren, R.; Zhao, Y.; Kasai, Y.; Miner, T.; Ries, R.E.; Lubman, O.; Fremont, D.H.; McLellan, M.D.; et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 2008, 111, 4797–4808.
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747.
- Drilon, A.; Li, G.; Dogan, S.; Gounder, M.; Shen, R.; Arcila, M.; Wang, L.; Hyman, D.M.; Hechtman, J.; Wei, G.; et al. What hides behind the MASC: Clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 920–926.
- Doebele, R.C.; Davis, L.E.; Vaishnavi, A.; Le, A.T.; Estrada-Bernal, A.; Keysar, S.; Jimeno, A.; Varella-Garcia, M.; Aisner, D.L.; Li, Y.; et al. An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101. Cancer Discov. 2015, 5, 1049–1057.
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018, 19, 705–714.
- Rolfo, C.; Ruiz, R.; Giovannetti, E.; Gil-Bazo, I.; Russo, A.; Passiglia, F.; Giallombardo, M.; Peeters, M.; Raez, L. Entrectinib: A potent new TRK, ROS1, and ALK inhibitor. Expert Opin. Investig. Drugs 2015, 24, 1493–1500.
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282.
- Murray, B.W.; Rogers, E.; Zhai, D.; Deng, W.; Chen, X.; Sprengeler, P.A.; Zhang, X.; Graber, A.; Reich, S.H.; Stopatschinskaja, S.; et al. Molecular Characteristics of Repotrectinib That Enable Potent Inhibition of TRK Fusion Proteins and Resistant Mutations. Mol. Cancer Ther. 2021, 20, 2446–2456.
- Cho, B.C.; Drilon, A.E.; Doebele, R.C.; Kim, D.W.; Lin, J.J.; Lee, J.; Ahn, M.J.; Zhu, V.W.; Ejadi, S.; Camidge, D.R. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J. Clin. Oncol. 2019, 37 (Suppl. 15), 9011.
- Hyman, D.; Kummar, S.; Farago, A.; Geoerger, B.; Mau-Sorensen, M.; Taylor, M.; Garralda, E.; Nagasubramanian, R.; Natheson, M.; Song, L.; et al. Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res. 2019, 79 (Suppl. 13), CT127.
- National Institutes of Health (NIH). ClinicalTrials.gov. A Study to Test the Safety of the Investigational Drug Selitrectinib in Children and Adults that May Treat Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03215511?cond=LOXO-195&rank=1 (accessed on 11 April 2019).
- Florou, V.; Nevala-Plagemann, C.; Whisenant, J.; Maeda, P.; Gilcrease, G.W.; Garrido-Laguna, I. Clinical Activity of Selitrectinib in a Patient with Mammary Analogue Secretory Carcinoma of the Parotid Gland with Secondary Resistance to Entrectinib. J. Natl. Compr. Cancer Netw. 2021, 19, 478–482.
- Katayama, R.; Gong, B.; Togashi, N.; Miyamoto, M.; Kiga, M.; Iwasaki, S.; Kamai, Y.; Tominaga, Y.; Takeda, Y.; Kagoshima, Y.; et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat. Commun. 2019, 10, 3604.
- Zhou, C.; Fan, H.; Wang, Y.; Wu, H.; Yang, N.; Li, K.; Wang, X.; Qin, X.; Yu, Q.; Fang, Y.; et al. Taletrectinib (AB-106; DS-6051b) in metastatic non-small cell lung cancer (NSCLC) patients with ROS1 fusion: Preliminary results of TRUST. J. Clin. Oncol. 2021, 39 (Suppl. 15).
- Kazandjian, D.; Blumenthal, G.M.; Luo, L.; He, K.; Fran, I.; Lemery, S.; Pazdur, R. Benefit-Risk Summary of Crizotinib for the Treatment of Patients with ROS1 Alteration-Positive, Metastatic Non-Small Cell Lung Cancer. Oncologist 2016, 21, 974–980.
- Singh, H.; Brave, M.; Beaver, J.A.; Cheng, J.; Tang, S.; Zahalka, E.; Palmby, T.R.; Venugopal, R.; Song, P.; Liu, Q.I.; et al. Food and Drug Administration Approval: Cabozantinib for the Treatment of Advanced Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 330–335.
- Zou, H.Y.; Li, Q.; Lee, J.H.; Arango, M.E.; McDonnell, S.R.; Yamazaki, S.; Koudriakova, T.B.; Alton, G.; Cui, J.J.; Kung, P.P.; et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007, 67, 4408–4417.
- Bowles, D.W.; Kessler, E.R.; Jimeno, A. Multi-targeted tyrosine kinase inhibitors in clinical development: Focus on XL-184 (cabozantinib). Drugs Today 2011, 47, 857–868.
- Shamroe, C.L.; Comeau, J.M. Ponatinib: A new tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann. Pharmacother. 2013, 47, 1540–1546.
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.S.; Xu, Q.; et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009, 16, 401–412.
- Karimi-Shah, B.A.; Chowdhury, B.A. Forced vital capacity in idiopathic pulmonary fibrosis--FDA review of pirfenidone and nintedanib. N. Engl. J. Med. 2015, 372, 1189–1191.
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008, 68, 4774–4782.
- Fuse, M.J.; Okada, K.; Oh-Hara, T.; Ogura, H.; Fujita, N.; Katayama, R. Mechanisms of Resistance to NTRK Inhibitors and Therapeutic Strategies in NTRK1-Rearranged Cancers. Mol. Cancer Ther. 2017, 16, 2130–2143.
- Russo, M.; Misale, S.; Wei, G.; Siravegna, G.; Crisafulli, G.; Lazzari, L.; Corti, G.; Rospo, G.; Novara, L.; Mussolin, B.; et al. Acquired Resistance to the TRK Inhibitor Entrectinib in Colorectal Cancer. Cancer Discov. 2016, 6, 36–44.
- Smeyne, R.J.; Klein, R.; Schnapp, A.; Long, L.K.; Bryant, S.; Lewin, A.; Lira, S.A.; Barbacid, M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994, 368, 246–249.
- Klein, R.; Smeyne, R.J.; Wurst, W.; Long, L.K.; Auerbach, B.A.; Joyner, A.L.; Barbacid, M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 1993, 75, 113–122.
- Klein, R.; Silos-Santiago, I.; Smeyne, R.J.; Lira, S.A.; Brambilla, R.; Bryant, S.; Zhang, L.; Snider, W.D.; Barbacid, M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 1994, 368, 249–251.
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 2, 1–12.
- Wang, H.; Li, Z.W.; Ou, Q.; Wu, X.; Nagasaka, M.; Shao, Y.; Ou, S.I.; Yang, Y. NTRK fusion positive colorectal cancer is a unique subset of CRC with high TMB and microsatellite instability. Cancer Med. 2022, 11, 2541–2549.
- Zhao, X.; Kotch, C.; Fox, E.; Surrey, L.F.; Wertheim, G.B.; Baloch, Z.W.; Lin, F.; Pillai, V.; Luo, M.; Kreiger, P.A.; et al. NTRK Fusions Identified in Pediatric Tumors: The Frequency, Fusion Partners, and Clinical Outcome. JCO Precis. Oncol. 2021, 1, 204–214.
- Pietrantonio, F.; Di Nicolantonio, F.; Schrock, A.B.; Lee, J.; Tejpar, S.; Sartore-Bianchi, A.; Hechtman, J.F.; Christiansen, J.; Novara, L.; Tebbutt, N.; et al. ALK, ROS1, and NTRK Rearrangements in Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djx089.
- Garralda, E.; Hong, D.S.; Xu, R.; Deeken, J.; Italiano, A.; Liu, T.; Ferrandiz, A.; Patel, J.; Lee, D.; Chung, H.; et al. Long-term efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase (TRK) fusion gastrointestinal (GI) cancer: An expanded dataset. Ann. Oncol. 2022, 33 (Suppl. 4), S370.
- Cohen, R.; Pudlarz, T.; Delattre, J.F.; Colle, R.; André, T. Molecular Targets for the Treatment of Metastatic Colorectal Cancer. Cancers 2020, 12, 2350.
- Forsythe, A.; Zhang, W.; Phillip Strauss, U.; Fellous, M.; Korei, M.; Keating, K. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther. Adv. Med. Oncol. 2020, 12, 1758835920975613.
- LiVolsi, V.A.; Abrosimov, A.A.; Bogdanova, T.; Fadda, G.; Hunt, J.L.; Ito, M.; Rosai, J.; Thomas, G.A.; Williams, E.D. The Chernobyl thyroid cancer experience: Pathology. Clin. Oncol. 2011, 23, 261–267.
- Taylor, J.; Pavlick, D.; Yoshimi, A.; Marcelus, C.; Chung, S.S.; Hechtman, J.F.; Benayed, R.; Cocco, E.; Durham, B.H.; Bitner, L.; et al. Oncogenic TRK fusions are amenable to inhibition in hematologic malignancies. J. Clin. Investig. 2018, 128, 3819–3825.
- Qin, K.; Hou, H.; Liang, Y.; Zhang, X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: A meta-analysis. BMC Cancer 2020, 20, 328.
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187.
- Agaram, N.P.; Zhang, L.; Sung, Y.S.; Chen, C.L.; Chung, C.T.; Antonescu, C.R.; Fletcher, C.D. Recurrent NTRK1 Gene Fusions Define a Novel Subset of Locally Aggressive Lipofibromatosis-like Neural Tumors. Am. J. Surg. Pathol. 2016, 40, 1407–1416.
- Kao, Y.C.; Fletcher, C.D.M.; Alaggio, R.; Wexler, L.; Zhang, L.; Sung, Y.S.; Orhan, D.; Chang, W.C.; Swanson, D.; Dickson, B.C.; et al. Recurrent BRAF Gene Fusions in a Subset of Pediatric Spindle Cell Sarcomas: Expanding the Genetic Spectrum of Tumors with Overlapping Features with Infantile Fibrosarcoma. Am. J. Surg. Pathol. 2018, 42, 28–38.
- Kiuru, M.; Jungbluth, A.; Kutzner, H.; Wiesner, T.; Busam, K.J. Spitz Tumors: Comparison of Histological Features in Relationship to Immunohistochemical Staining for ALK and NTRK1. Int. J. Surg. Pathol. 2016, 24, 200–206.
- Uguen, A. Spitz Tumors with NTRK1 Fusions: TRK-A and pan-TRK Immunohistochemistry as Ancillary Diagnostic Tools. Am. J. Surg. Pathol. 2019, 43, 1438–1439.
- Yeh, I.; Tee, M.K.; Botton, T.; Shain, A.H.; Sparatta, A.J.; Gagnon, A.; Vemula, S.S.; Garrido, M.C.; Nakamaru, K.; Isoyama, T.; et al. NTRK3 kinase fusions in Spitz tumours. J. Pathol. 2016, 240, 282–290.
- Silvertown, J.D.; Lisle, C.; Semenuk, L.; Knapp, C.; Jaynes, J.; Berg, D.; Kaul, N.; Lachapelle, J.; Richardson, L.; Speevak, M.; et al. Prevalence of NTRK Fusions in Canadian Solid Tumour Cancer Patients. Mol. Diagn. Ther. 2023, 27, 87–103.
