Various techniques for extracorporeal blood purification can decrease levels of elevated proinflammatory cytokines in septic shock, potentially mitigating the severity of the systemic inflammatory response. Some methods are effective in removing endotoxins, especially in sepsis caused by Gram-negative bacteria, which may aid in stabilizing the patient’s condition. Blood purification can enhance hemodynamic stability and reduce the need for vasopressors, crucial for managing septic shock. Techniques like continuous renal replacement therapy (CRRT) offer simultaneous management of acute kidney injury—a frequent complication in septic shock—alongside the removal of toxins and cytokines.
- sepsis
- septic hyperinflammation
- blood purification
- immune response
- cytokines
- endotoxi
1. Introduction
1.1. Septic Hyperinflammation
1.2. Immune Response Mechanisms in Sepsis: From Recognition to Regulation
1.3. Neutrophils in Sepsis: Roles in Defense, Hyperinflammation, and Organ Damage
1.4. Endothelial Dysfunction and Thromboinflammation in Hyperinflammatory Diseases
1.5. Complement System Activation and Immunothrombosis in Sepsis and Systemic Inflammation
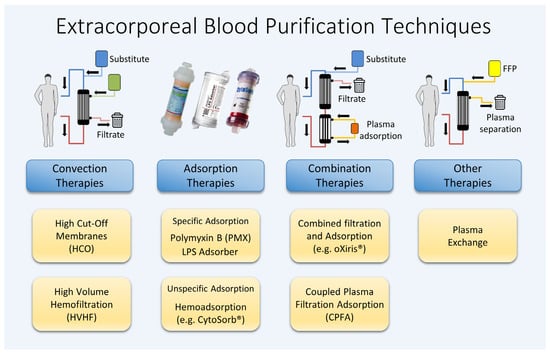
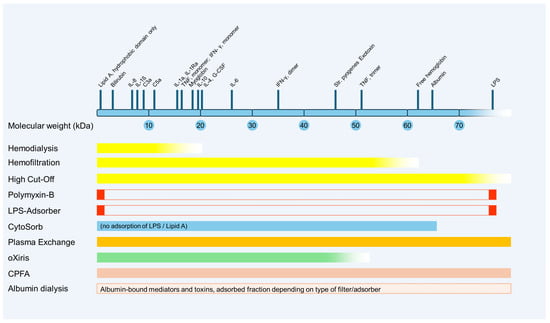
2. Renal Replacement Therapies (RRTs)
2.1. High-Cut-Off Membranes
2.2. High-Volume Hemofiltration
3. Adsorption
3.1. Polymyxin B-Immobilized Fiber Columns (Specific Hemoadsorption)
3.2. LPS Adsorber
3.3. CytoSorb® (Unspecific Hemadsorption)
4. Therapeutic Plasma Exchange (TPE)
5. Combination Methods
5.1. oXiris®
5.2. Coupled Plasma Filtration Adsorption (CPFA)
6. Albumin Dialysis
This entry is adapted from the peer-reviewed paper 10.3390/ijms25063120
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211.
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528.
- Kemball, C.C.; Alirezaei, M.; Whitton, J.L. Type B coxsackieviruses and their interactions with the innate and adaptive immune systems. Future Microbiol. 2010, 5, 1329–1347.
- Tamayo, E.; Fernandez, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gomez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011, 22, 82–87.
- Tang, B.M.; Huang, S.J.; McLean, A.S. Genome-wide transcription profiling of human sepsis: A systematic review. Crit. Care 2010, 14, R237.
- Drifte, G.; Dunn-Siegrist, I.; Tissieres, P.; Pugin, J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013, 41, 820–832.
- Nierhaus, A.; Linssen, J.; Wichmann, D.; Braune, S.; Kluge, S. Use of a weighted, automated analysis of the differential blood count to differentiate sepsis from non-infectious systemic inflammation: The intensive care infection score (ICIS). Inflamm. Allergy Drug Targets 2012, 11, 109–115.
- Nierhaus, A.; Klatte, S.; Linssen, J.; Eismann, N.M.; Wichmann, D.; Hedke, J.; Braune, S.A.; Kluge, S. Revisiting the white blood cell count: Immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis—A prospective, observational study. BMC Immunol. 2013, 14, 8.
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85.
- Cox, L.E.; Walstein, K.; Vollger, L.; Reuner, F.; Bick, A.; Dotsch, A.; Engler, A.; Peters, J.; von Kockritz-Blickwede, M.; Schafer, S.T. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15.
- Ortmann, W.; Kolaczkowska, E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018, 371, 473–488.
- Camicia, G.; Pozner, R.; de Larranaga, G. Neutrophil extracellular traps in sepsis. Shock 2014, 42, 286–294.
- Daix, T.; Guerin, E.; Tavernier, E.; Mercier, E.; Gissot, V.; Herault, O.; Mira, J.P.; Dumas, F.; Chapuis, N.; Guitton, C.; et al. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest 2018, 154, 617–627.
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111.
- Fernandez-Sarmiento, J.; Salazar-Pelaez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300.
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087.
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762.
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725.
- Denk, S.; Taylor, R.P.; Wiegner, R.; Cook, E.M.; Lindorfer, M.A.; Pfeiffer, K.; Paschke, S.; Eiseler, T.; Weiss, M.; Barth, E.; et al. Complement C5a-Induced Changes in Neutrophil Morphology During Inflammation. Scand. J. Immunol. 2017, 86, 143–155.
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M.; Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330.
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 89–95.
- Morgera, S.; Slowinski, T.; Melzer, C.; Sobottke, V.; Vargas-Hein, O.; Volk, T.; Zuckermann-Becker, H.; Wegner, B.; Muller, J.M.; Baumann, G.; et al. Renal replacement therapy with high-cutoff hemofilters: Impact of convection and diffusion on cytokine clearances and protein status. Am. J. Kidney Dis. 2004, 43, 444–453.
- Atari, R.; Peck, L.; Visvanathan, K.; Skinner, N.; Eastwood, G.; Bellomo, R.; Storr, M.; Goehl, H. High cut-off hemofiltration versus standard hemofiltration: Effect on plasma cytokines. Int. J. Artif. Organs 2016, 39, 479–486.
- Honore, P.M.; Jacobs, R.; Boer, W.; Joannes-Boyau, O.; De Regt, J.; De Waele, E.; Van Gorp, V.; Collin, V.; Spapen, H.D. New insights regarding rationale, therapeutic target and dose of hemofiltration and hybrid therapies in septic acute kidney injury. Blood Purif. 2012, 33, 44–51.
- Villa, G.; Neri, M.; Bellomo, R.; Cerda, J.; De Gaudio, A.R.; De Rosa, S.; Garzotto, F.; Honore, P.M.; Kellum, J.; Lorenzin, A.; et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: Practical applications. Crit. Care 2016, 20, 283.
- Joannes-Boyau, O.; Honore, P.M.; Perez, P.; Bagshaw, S.M.; Grand, H.; Canivet, J.L.; Dewitte, A.; Flamens, C.; Pujol, W.; Grandoulier, A.S.; et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): A multicentre randomized controlled trial. Intensive Care Med. 2013, 39, 1535–1546.
- Network, V.N.A.R.F.T.; Palevsky, P.M.; Zhang, J.H.; O’Connor, T.Z.; Chertow, G.M.; Crowley, S.T.; Choudhury, D.; Finkel, K.; Kellum, J.A.; Paganini, E.; et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 2008, 359, 7–20.
- Investigators, R.R.T.S.; Bellomo, R.; Cass, A.; Cole, L.; Finfer, S.; Gallagher, M.; Lo, S.; McArthur, C.; McGuinness, S.; Myburgh, J.; et al. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 2009, 361, 1627–1638.
- Zhang, P.; Yang, Y.; Lv, R.; Zhang, Y.; Xie, W.; Chen, J. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: A single-center randomized clinical trial. Nephrol. Dial. Transplant. 2012, 27, 967–973.
- Junhai, Z.; Beibei, C.; Jing, Y.; Li, L. Effect of High-Volume Hemofiltration in Critically Ill Patients: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2019, 25, 3964–3975.
- Yaroustovsky, M.; Plyushch, M.; Popov, D.; Samsonova, N.; Abramyan, M.; Popok, Z.; Krotenko, N. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. J. Inflamm. 2013, 10, 8.
- Ikeda, T.; Kamohara, H.; Suda, S.; Nagura, T.; Tomino, M.; Sugi, M.; Wajima, Z. Comparative Evaluation of Endotoxin Activity Level and Various Biomarkers for Infection and Outcome of ICU-Admitted Patients. Biomedicines 2019, 7, 47.
- Vincent, J.L.; Laterre, P.F.; Cohen, J.; Burchardi, H.; Bruining, H.; Lerma, F.A.; Wittebole, X.; De Backer, D.; Brett, S.; Marzo, D.; et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005, 23, 400–405.
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Bobbio Pallavicini, F.M.; et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009, 301, 2445–2452.
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med. 2015, 41, 975–984.
- Lipcsey, M.; Tenhunen, J.; Pischke, S.E.; Kuitunen, A.; Flaatten, H.; De Geer, L.; Sjolin, J.; Frithiof, R.; Chew, M.S.; Bendel, S.; et al. Endotoxin Removal in Septic Shock with the Alteco LPS Adsorber Was Safe But Showed no Benefit Compared to Placebo in the Double-Blind Randomized Controlled Trial-the Asset Study. Shock 2020, 54, 224–231.
- Schädler, D.; Porzelius, C.; Jörres, A.; Marx, G.; Meier-Hellmann, A.; Putensen, C.; Quintel, M.; Spies, C.; Engel, C.; Weiler, N.; et al. A multicenter randomized controlled study of an extracorporeal cytokine hemoadsorption device in septic patients. Crit. Care 2013, 17, P62.
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Druner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74.
- David, S.; Stahl, K. To remove and replace-a role for plasma exchange in counterbalancing the host response in sepsis. Crit. Care 2019, 23, 14.
- Bockmeyer, C.L.; Claus, R.A.; Budde, U.; Kentouche, K.; Schneppenheim, R.; Losche, W.; Reinhart, K.; Brunkhorst, F.M. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica 2008, 93, 137–140.
- Azfar, M.F.; Khan, M.F.; Habib, S.S.; Aseri, Z.A.; Zubaidi, A.M.; Aguila, D.O.; Suriya, M.O.; Ullah, H. Prognostic value of ADAMTS13 in patients with severe sepsis and septic shock. Clin. Investig. Med. 2017, 40, E49–E58.
- Broman, M.E.; Hansson, F.; Vincent, J.L.; Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS ONE 2019, 14, e0220444.
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12.
- Monard, C.; Rimmele, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019, 47 (Suppl. S3), 1–14.
- Schwindenhammer, V.; Girardot, T.; Chaulier, K.; Gregoire, A.; Monard, C.; Huriaux, L.; Illinger, J.; Leray, V.; Uberti, T.; Crozon-Clauzel, J.; et al. oXiris(R) Use in Septic Shock: Experience of Two French Centres. Blood Purif. 2019, 47 (Suppl. S3), 1–7.
- Tetta, C.; Cavaillon, J.M.; Schulze, M.; Ronco, C.; Ghezzi, P.M.; Camussi, G.; Serra, A.M.; Curti, F.; Lonnemann, G. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol. Dial. Transplant. 1998, 13, 1458–1464.
- Alcaraz-Quiles, J.; Casulleras, M.; Oettl, K.; Titos, E.; Flores-Costa, R.; Duran-Guell, M.; Lopez-Vicario, C.; Pavesi, M.; Stauber, R.E.; Arroyo, V.; et al. Oxidized Albumin Triggers a Cytokine Storm in Leukocytes Through P38 Mitogen-Activated Protein Kinase: Role in Systemic Inflammation in Decompensated Cirrhosis. Hepatology 2018, 68, 1937–1952.
- Casulleras, M.; Flores-Costa, R.; Duran-Guell, M.; Alcaraz-Quiles, J.; Sanz, S.; Titos, E.; Lopez-Vicario, C.; Fernandez, J.; Horrillo, R.; Costa, M.; et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci. Transl. Med. 2020, 12, eaax5135.
- Schmuck, R.B.; Nawrot, G.H.; Fikatas, P.; Reutzel-Selke, A.; Pratschke, J.; Sauer, I.M. Single Pass Albumin Dialysis-A Dose-Finding Study to Define Optimal Albumin Concentration and Dialysate Flow. Artif. Organs 2017, 41, 153–161.
