Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Photocatalytic synthesis of H2O2 has emerged as a compelling alternative, offering the prospect of harnessing solar energy directly to drive chemical reactions, thereby circumventing the need for energy-intensive processes and deleterious chemicals.
- covalent organic framework
- H2O2 photosynthesis
- photosynthesis
1. Introduction
The quest for sustainable and clean energy sources has become a paramount concern in the face of escalating global energy demands and the pressing challenges of climate change. Solar energy, being abundant and renewable, stands out as a promising candidate to address these challenges. Efficiently harnessing the vast potential of solar energy not only offers a solution to the impending energy crisis but also holds the promise of reducing the carbon footprint associated with conventional fossil fuels. Photocatalysis is a process that uses light to drive chemical reactions and has emerged as an important technology [1,2,3,4,5,6]. By converting solar energy into chemical energy, photocatalysis offers a dual advantage [4,7]: it provides an avenue for sustainable energy storage and paves the way for the synthesis of valuable chemicals, including H2, CO, and hydrogen peroxide (H2O2).
Among various chemicals synthesized by photosynthesis, H2O2 has attracted significant attention [2,8,9,10]. H2O2 is valuable for its multifaceted applications and environmentally amicable nature, and has become an indispensable chemical field like industrial processes, environmental remediation, and healthcare and medical applications [11,12,13,14]. Its ability to decompose into water and oxygen underpins its appeal as an eco-friendly oxidant, minimizing the risk of generating secondary pollutants. The conventional anthraquinone oxidation process [15], which has been the industrial applicable for H2O2 production, is increasingly being scrutinized for its inherent drawbacks. These include not only the substantial energy consumption and the utilization of hazardous substrates but also the generation of significant amounts of waste, which poses considerable environmental and economic challenges. In light of these limitations, the researchers have pivoted towards seeking alternative, sustainable, and cleaner methods for H2O2 production, with a particular emphasis on minimizing environmental repercussions. Photocatalytic synthesis of H2O2 has emerged as a compelling alternative, offering the prospect of harnessing solar energy directly to drive chemical reactions, thereby circumventing the need for energy-intensive processes and deleterious chemicals [16,17]. This approach not only aligns with the global shift towards sustainable energy but also presents a pathway for the localized, on-demand production of H2O2, reducing the need for storage and transportation.
In the realm of photocatalytic H2O2 synthesis, the role of catalysts is paramount, dictating the efficiency, selectivity, and stability of the photocatalytic processes. Conventional photocatalysts are predominantly based on precious metals such as platinum [18], palladium [19], and gold [20,21], demonstrating commendable performance in facilitating the production of H2O2. However, the deployment of these noble metal-based catalysts is significantly hampered by their scarcity in the Earth’s crust, which intrinsically leads to high costs and poses sustainability concerns, especially in the context of large-scale applications and global accessibility. Consequently, the exploration of alternative non-metal-based photocatalysts has become a focal point in contemporary research, aiming to circumvent the limitations associated with noble-metal catalysts. Metal-free photocatalysts, particularly those based on linear polymers [22], polymeric carbon nitride (PCN) [23,24], polymer resins [25], supramolecular coordination [26,27], and covalent organic frameworks (COFs) [2,28,29], have emerged as promising candidates, offering the advantages of abundance and low cost under photocatalytic conditions. Among these, COFs, with their intrinsic porosity, tunable structures, and the ability to incorporate a myriad of organic functional groups (Figure 1), have garnered substantial attention since 2020 [30,31,32].
Figure 1. The advantages of COFs for the photosynthesis of H2O2.
COFs are typically a class of porous polymers formed by organic building blocks connected through covalent bonds. They were first synthesized by Yaghi et al. under solvothermal conditions through the self-condensation of phenyl diboronic acid (PDBA) and the co-condensation of PDBA with hexahydroxytriphenylene (HHTP) [33]. This work opened the door to COF research. Subsequently, various methods for synthesizing COFs have been reported, including solvothermal [34,35,36,37,38], microwave [39,40,41], ionothermal [42,43,44], and mechanochemical methods [45,46] for powder synthesis, and interfacial methods [47,48,49] for thin-film synthesis. At the same time, a wide variety of organic building blocks and linkages have been reported. To date, reported linkages include boroxine [50], boronate-ester [51,52,53], imine [54,55,56,57], hydrazone [58,59], squaraine [60,61,62], azine [63,64,65], imide [66,67], C=C [68,69,70,71], 1,4-dioxin linkage [72,73], among others. The COFs synthesized by these methods have shown great potential in applications such as sensing [74,75,76], catalysis [5,77,78,79], energy storage and conversion, [6,80,81,82,83] organic electronic devices [84,85,86,87,88,89,90,91,92], etc. [6,80,81,82,83,93,94,95,96].
2. Principles of Photocatalytic H2O2 Generation
Equation (1) illustrates the full process of photocatalytic synthesis of H2O2. This procedure encompasses two distinct half-reactions, namely the oxygen reduction reaction (ORR) (Equations (2) and (3)) and the water oxidation reaction (WOR) (Equation (4)). In response to the impetus of photons, the electrons within the catalyst undergo a transition from the valence band (VB) to the conduction band (CB), thus giving rise to the emergence of photo-excited h+ and e− species. The subsequent migration of these charges to the catalyst’s surface facilitates their active participation in a cascading series of reduction-oxidation reactions (Figure 2a), thereby selectively yielding H2O2.
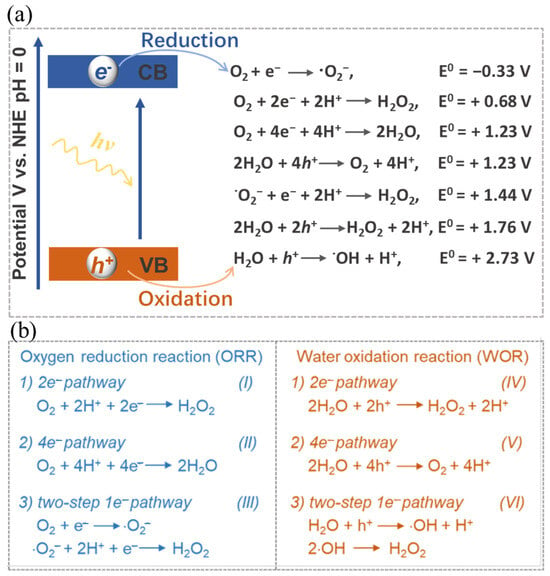
Figure 2. (a) Schematic illustration and (b) Corresponding energy diagrams of the oxygen reduction and water oxidation involved in H2O2 photosynthesis. Reproduced with permission [1].
The photo-excited e− with reducibility is capable of reacting with oxygen through a 2e− ORR process for the generation of H2O2 (see Equation (2)). It is worth noting that the oxygen involved in this reaction may be derived from either the 4e− WOR or the atmospheric oxygen. Theoretically, as shown in Figure 2b, the 2e− ORR can proceed directly through a 2e− process (O2 + 2e− + 2H+ → H2O2) or two consecutive 1e− reactions via the superoxide radical intermediate (·O2−) (III of Figure 2b). In parallel, the photo-excited h+, which exhibits oxidizability, enables water molecules to generate H2O2 through a directly one-step 2e− WOR (see Equation (3)) or two-step 1e− WOR, or to produce O2 through a 4e− WOR (see Equation (4)). Similar to the two-step 1e− ORR process, in the two-step 1e− WOR process, the photo-induced proton h+ can initially oxidize H2O to generate a hydroxyl radical (·OH) intermediate. Subsequently, H2O2 is formed indirectly through the combination of two ·OH (VI of Figure 2b). These intermediates can be identified through in-situ characterization techniques. In-situ diffuse reflectance infrared Fourier transform spectroscopy is extensively utilized for the characterization of intermediates in the photocatalytic generation of H2O2. A well-designed photocatalyst aimed at the overall reaction should possess functional groups that effectively promote both the 2e− ORR and 2e− WOR. These two reactions, in turn, serve to maintain charge balance by, respectively, consuming e− and h+. In instances where catalysts are specifically engaged in one of the half-reactions, sacrificial reagents are often employed to consume uninvolved charges, thereby safeguarding the catalyst and directing the reaction’s selectivity.

Nonetheless, it is worth noting that the solar-to-chemical energy conversion efficiency (SCC) in the context of photocatalytic H2O2 synthesis remains relatively low at present, seldom surpassing 1% [97]. This efficiency discrepancy is quite pronounced when compared to the performance observed in photocatalytic hydrogen production from water [98,99]. Several factors contribute to this relatively low efficiency, including limited light absorption, a propensity for charge recombination, and challenges in achieving desirable selectivity towards H2O2 [97,100,101]. Particularly, achieving selectivity towards H2O2 presents a significant hurdle (Figure 2). The WOR process commonly tends to favor O2 production via the 4e− pathway rather than H2O2 production through the 2e− pathway [17]. Additionally, side reactions and H2O2 decomposition also impact both the yield and selectivity [102,103]. Through the desirable optimization of catalyst structures and reaction conditions, significant enhancements can be achieved in terms of the yield and selectivity of photocatalytic H2O2 synthesis.
This entry is adapted from the peer-reviewed paper 10.3390/polym16050659
This entry is offline, you can click here to edit this entry!
