Mesenchymal stem/stromal cells (MSCs) are multipotent cells located in different areas of the human body. The oral cavity is considered a potential source of MSCs because they have been identified in several dental tissues (D-MSCs). Clinical trials in which cells from these sources were used have shown that they are effective and safe as treatments for tissue regeneration. Importantly, immunoregulatory capacity has been observed in all of these populations. Since this property is of clinical interest for cell therapy protocols, it is relevant to analyze the differences in immunoregulatory capacity, as well as the mechanisms used by each type of MSC. Interestingly, D-MSCs are the most suitable source for regenerating mineralized tissues in the oral region. Furthermore, the clinical potential of D-MSCs is supported due to their adequate capacity for proliferation, migration, and differentiation. There is also evidence for their potential application in protocols against autoimmune diseases and other inflammatory conditions due to their immunosuppressive capacity.
1. Introduction
Mesenchymal stem/stromal cells (MSCs) were first discovered in the bone marrow (BM-MSCs) of guinea pigs more than 50 years ago by Friedenstein and his collaborators [
1]. The International Society for Cell Therapy defines these cells as adherent cells with a fibroblast morphology that simultaneously express the immunophenotypic markers CD105, CD90, and CD73, present low levels of human leukocyte antigen (HLA) class I molecules and lack HLA class II molecules and markers of hematopoietic and endothelial cells. More recently, new surface molecules have been discovered, and MSCs are now recognized as cells that express STRO-1, CD106, and CD146. Finally, MSCs can differentiate when cultured in a specific inducing medium, at least in adipocytes, chondroblasts, and osteoblasts [
2].
BM-MSCs are characterized by regenerative and immunomodulatory properties that can be exploited in clinical treatments for various immune diseases [
3,
4]. Unfortunately, obtaining them from this source presents drawbacks that reduce their feasibility for use in cell therapy as a result of the painful and invasive process involved [
5]. The biological potential and number of cells may vary or decrease with the sex and age of the donor [
6,
7,
8,
9,
10,
11]. When cultivated in the long term, proliferation is negatively affected [
12], as are the morphology, differentiation capacity, and genetic stability [
13]. Finally, the anti-inflammatory potential of these cells may be compromised when they are obtained from donors with some pathological conditions, such as rheumatoid arthritis [
14], which would reduce the possibility of using autologous samples. For these reasons, alternative sources to BM where these inconveniences are minimized are needed.
Currently, MSCs can be obtained from neonatal tissues, including umbilical cord blood (UCB) [
15], the umbilical cord (UC), and the placenta [
16], as well as adult sources, such as adipose tissue (AT) [
17], synovial fluid [
18], the skin [
19], the lungs [
20], the liver [
21], peripheral blood [
22,
23,
24,
25], and dental tissues [
26]. Most MSCs from dental tissues, possess similar properties to MSCs from BM; however, there are still some gaps in the knowledge of their biological characteristics.
2. Mesenchymal Stromal Cells from Dental and Periodontal Tissues
The teeth are essential multifunctional appendages for functions such as speaking or eating [
27]. They are divided into two main regions: the upper part or crown and the root, which is anchored within the mandibular and maxillary bones. In humans, there are two sets of teeth: the initial deciduous or primary teeth and the successive permanent (secondary) teeth [
28].
Structurally, teeth are made up of three highly mineralized tissues called enamel, dentin, and cement, whose functions include providing support, size, shape, and anchorage [
29,
30]. Enamel and dentin also protect a fourth nonmineralized tissue known as the dental pulp. This area serves as a reservoir for fibroblasts and is characterized by a vascular–nervous system that features a unique combination of blood vessels, nerves, odontoblasts, and an extracellular matrix [
31,
32]. The vascular–nervous system plays a fundamental role in tooth function, feeding it from the apex without accessory vascularization. Unlike other oral tissues adjacent to the tooth, this mechanism maintains homeostasis and provides the tooth with neurosensory function, aiding in repair processes [
27,
33,
34]. On the other hand, teeth also rely on other tissues, including the periodontal ligament, the alveolar bone, and the gingival tissue; together, these tissues are known as periodontal tissues or simply the periodontium.
The functions of the periodontium include supporting the tooth and protecting it against invasion by oral microorganisms; it is essential in the immune response and allows the attachment of the tooth to the bone [
35,
36].
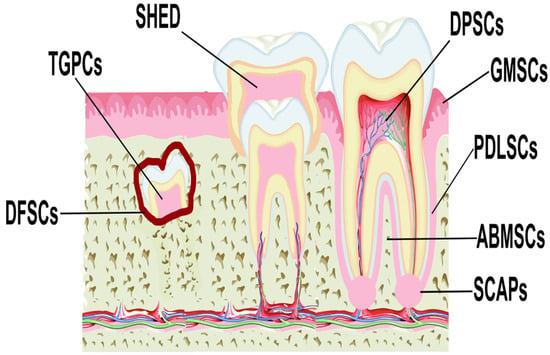
MSCs have been isolated from various anatomical regions of the oral cavity (
Figure 1), including the dental pulp (DPSCs) [
37,
38], periodontal ligament (PDLSCs) [
39,
40], gingival tissue (GMSCs) [
41,
42], apical papilla (SCAPs) [
43,
44], dental follicle (DFSCs) [
45,
46], human exfoliated deciduous teeth (SHED) [
47,
48], alveolar bone (ABMSCs), and tooth germ (TGPCs) [
34], and these are collectively defined as dental MSCs (D-MSCs) [
49].
Figure 1. Locations of different sources of MSCs derived from dental tissues. Abbreviations: DPSCs, dental pulp stem cells; PDLSCs, periodontal ligament stem cells; GMSCs, gingiva-derived MSCs; SCAPs, stem cells from the apical papilla; DFSCs, dental follicle stem cells; SHED, stem cells from exfoliated deciduous teeth; ABMSCs, alveolar bone-derived MSCs; TGPCs, tooth germ progenitor cells.
Dental tissues are frequently obtained during routine dental extraction procedures. The dental pieces and/or tissues are kept in phosphate-buffered solutions, usually supplemented with antibiotics and antifungals, and a series of washes with fresh solution are performed to remove unwanted tissues. The tissues of interest are stored at 4 °C before beginning the procedures to obtain MSCs. To access and obtain the dental pulp, the teeth are crushed or the crowns are cut. Other tissues such as the ligament, gingiva, and apical papilla can be separated mechanically by using forceps [
50,
51,
52,
53,
54,
55].
The most common methods used to obtain MSCs are explant and enzymatic digestion. In the first method, the tissues obtained are crushed into small pieces of 1 mm and placed on plates with ideal culture medium for the growth of these cells supplemented with fetal bovine serum and antibiotics. They are then incubated at 37 °C and 5% CO
2. Additionally, in some procedures, a coverslip can be added over the explants to prevent the tissues from moving during the culture time. On the other hand, enzymatic digestion is carried out through the incubation of the crushed tissues in collagenase I and dispase II. The incubation time can vary from 30 min to 2 h at 37 °C. Once digestion has been carried out, the cells are seeded on plates with the same culture conditions mentioned above. Once the cells adhere, the medium and excess tissues are removed and fresh medium is added to continue cell expansion, and subsequently, the capacity to generate colonies, morphology, immunophenotype, and the differentiation potential characteristic of the MSCs are evaluated [
50,
51,
52,
53,
54,
55]. Although most protocols use enzymatic digestion to obtain D-MSCs, both methods are reliable. They can obtain a high number of cells and there is no solid evidence that either affects the biological properties of the cells. However, isolation methods continue to improve with the purpose of obtaining the greatest number of cells with the necessary quality required to be able to use them in preclinical and clinical research.
D-MSCs have several similar characteristics which are summarized in
Table 1; for example, they come from easily accessible tissues, they exhibit fibroblast morphology, they provide a high number of MSCs with high proliferation rates, and, unlike MSCs from other sources, they maintain their qualities for more passages [
49,
56,
57,
58,
59]. However, differences have been observed in other characteristics, such as the immunophenotype, where the expression of some markers may vary. In addition, although they have similar multipotentialities for differentiation toward chondrocytes and osteoblasts, their adipogenic capacity is lower than that of other tissues, such as BM [
58,
60,
61,
62,
63,
64]. Moreover, D-MSCs derived from neural crest cells possess an increased potential for differentiation into neural cells [
65,
66,
67,
68]. Taken together, these findings indicate that although all of these sources originate in related anatomical regions, the biological properties of each can vary, and of particular importance is their immunosuppressive capacity due to their relevance in clinical protocols. In the case of D-MSCs, they have great therapeutic potential for applications in tissue repair, including that of bone, dental, and soft tissues.
Table 1. Characteristics of D-MSCs.
| Source |
Efficiency of Isolation |
Surface Markers |
Embryonic
Markers |
Neural Markers |
Differentiation Potential |
| DPSCs |
++++ |
CD13, CD29, CD44, CD59, CD73, CD90, CD105, CD146 |
STRO-1, OCT-4, Nanog, SSEA-1, SEEA-4, SOX-2 |
β3-tubulin, NFM, Nestin, CNPase, S100, CD271 |
Adipogenic, osteogenic, odontoblast, angiogenic, and neuronal cells |
| PDLSCs |
++++ |
CD10, CD29, CD44, CD73, CD105 |
SSEA-1, SSEA-3, SSEA-4, TRA-1–60, TRA-1–81, OCT-4,
Nanog, SOX-2, REX1, ALP |
Nestin, OCT-4, SSEA-4, CD271, SOX-10 |
Adipogenic, chondrogenic, osteogenic, and neuronal cells |
| GMSCs |
++++ |
CD73, CD90, CD105 |
SSEA-4, OCT-4, Nanog |
Nestin, SOX10, β3-tubulin, NFM, CNPase |
Adipogenic, chondrogenic, osteogenic, angiogenic, and neuronal cells |
| SCAPs |
+++ |
CD24, CD44, CD90, CD146, STRO-1 |
OCT-4, Nanog, NOTCH-1, SOX-2 |
OCT-4, SOX2, Nestin |
Adipogenic, chondrogenic osteogenic, odontogenic, and neuronal cells |
| DFSCs |
++ |
CD13, CD29, CD44, CD56, CD59, CD90, CD105, CD106, CD166, STRO-1 |
OCT-4, Nanog, NOTCH-1, SOX-2 |
OCT-4, SOX2, Nestin |
Osteogenic, odontogenic, and cementogenic |
| SHED |
+++ |
CD29, CD73, CD90, CD146, CD166 |
OCT-4, Nanog, SSEA-3, SSEA-4, NOTCH-1, SOX-2 |
β3-tubulin, NFM, Nestin, CNPase, GAD, NeuN, GFAP, CD271, Vimentin, OCT-4, PAX-6, NSE, MAP-2, PSA- NCAM |
Adipogenic, chondrogenic, osteogenic, odontogenic, angiogenic, and neuronal cells |
| ABMSCs |
++++ |
CD73, CD90, CD105, STRO-1 |
Oct4, KLF4, Sox2, cMyc |
NF-M, NeuN, GFAP |
Adipogenic, chondrogenic, and osteogenic |
| TGPCs |
+ |
CD29, CD73, CD90, CD105, CD166 |
Nanog, OCT-4, SOX-2, Klf4, c-Myc |
Nestin |
Adipogenic, chondrogenic, osteogenic, and neuronal cells |
This entry is adapted from the peer-reviewed paper 10.3390/ijms25041986