Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Fibroblast activation protein (FAP) is a serine protease characterized by its high expression in cancer-associated fibroblasts (CAFs) and near absence in adult normal tissues and benign lesions. This unique expression pattern positions FAP as a prospective biomarker for targeted tumor radiodiagnosis and therapy. The advent of FAP-based radiotheranostics is anticipated to revolutionize cancer management.
- FAP
- radiopharmaceuticals
- fibroblast activation protein
1. Introduction
Cancer is a heterogeneous disease that develops in an incredibly complex microenvironment [1][2][3][4][5][6]. This microenvironment encompasses not only cancerous cells but also vital constituents like the extracellular matrix [7][8][9][10], stromal cells [11][12][13], and immune cells [14][15][16], forming the tumor microenvironment (TME) [17][18][19][20]. The TME, an intricate milieu unique to tumors, exerts profound influences on tumor progression, immune evasion, metastasis, and therapeutic resistance [4][21][22][23][24][25][26].
Among the various types of fibroblasts implicated in cancer, cancer-associated fibroblasts (CAFs) stand out prominently [27][28][29][30][31][32][33][34]. These CAFs constitute a staggering 80% of all fibroblasts within the TME, assuming a pivotal role as oncogenic regulators with far-reaching effects on tumor cell proliferation, migration, extracellular matrix remodeling, and immunosuppression [35][36][37][38][39][40][41]. Originating from fibroblasts in normal tissues [42][43][44][45], these cells undergo a reversible transformation akin to myofibroblasts after injury, actively participating in wound healing [27][46][47][48][49]. Myofibroblasts transition from their initial presence in granulation tissue to become the predominant cell type in the proliferative phase, ultimately diminishing as the wound healing process concludes [50][51][52]. In the context of cancer, fibroblasts and other stromal cells orchestrate this transformation into CAFs by secreting transforming growth factors in the TME [27][46][53][54]. Notably, CAFs, in contrast to cancer cells, exhibit remarkable stability and resistance to drug resistance, underscoring their viability as a pivotal biological target for cancer diagnosis and therapy [55][56][57][58].
Identification of specific biomarkers on the surface of CAFs offers a strategic avenue for targeted radiological diagnostics and therapeutics [59][60][61][62][63][64]. Among these biomarkers, fibroblast activation protein (FAP) has gained widespread attention for its potential in CAF identification and targeting [65][66][67][68][69][70][71]. FAP, a member of the dipeptidyl peptidase 4 (DPP4) family, boasts a molecular weight of 170 kDa [72][73][74][75][76]. It assumes the guise of a type II transmembrane serine protease, typically existing as a homodimer [77][78][79]. Functionally, FAP exhibits dipeptidyl peptidase and endopeptidase activities [65][80][81][82], and its significance extends to normal embryonic development and tissue modeling [83][84][85]. Remarkably, FAP remains scarcely noticeable or entirely absent in normal adult tissues [86][87]. However, it undergoes marked upregulation during processes such as wound healing, atherosclerotic plaque formation, and fibrosis [88][89][90][91], and prominently features in over 90% of human epithelial carcinomas [72][80][92][93][94][95][96][97][98].
2. Antibody-Based Radiopharmaceuticals Targeting FAP
2.1. Iodine-131-Labeled Monoclonal Antibody F19
The discovery of FAP, a type II transmembrane serine protease, can be traced back to 1986 when it was initially identified as the F19 antigen during studies involving cultured fibroblasts and the monoclonal antibody (mAb) F19 [99][100][101]. Subsequently, in 1994, the surface antigen expressed by F19 cells was officially named FAP [86][102]. In 1990, Garin-Chesa et al. proposed that in the context of cancer, epithelial cancer, F19+ fibroblasts, colloquially referred to as FAP, emerged as a consistent molecular trait of the reactive stroma. The role of mAbF19 in their identification was pivotal [94]. Human FAP, discerned through mAbF19, became a prominent cell surface antigen [103]. Due to its abundant presence within the tumor mesenchyme, FAP can serve as a target for radionuclide antibody conjugates in cancer patients [103][104][105].
In 1988, Old et al. conducted a comprehensive examination of six human cell surface glycoproteins, each defined by mAb, with the intention of characterizing the surface phenotype of cultivated mesenchymal cells [92]. Among these antibodies, mAbF19 effectively identified glycoproteins with molecular weights of 120,000 and 95,000, expressed on cultivated fibroblasts and a proportion of sarcoma cell lines, respectively. This discovery marked mAbF19 as a superior antibody for these purposes compared with other candidates, such as mAbF24, G171, G253, and K117. Another antibody, S5, exhibited expression patterns similar to mAbF19 but had limited in vivo expression [92]. It became evident that the fibroblasts surrounding tumor cells offer effective targets for cancer immunolocalization or immunotherapy, owing to their recognition by mAbF19 [106][107][108].
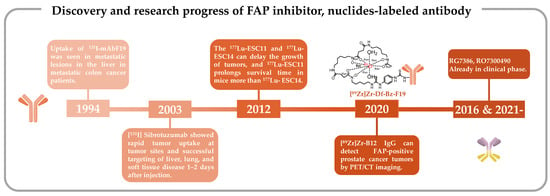
The pioneering clinical study involving FAP-targeting radiopharmaceuticals utilized 131I-labeled mAbF19 for tumor imaging in patients with liver metastases from colon cancer [109]. Welt et al. conducted this study in 1994, wherein 17 patients scheduled for resection of localized metastases or regional chemotherapy received intravenous administration of 131I-mAbF19. Imaging results from this series of studies revealed that the tumor-to-normal tissue ratio reached its peak after 3–5 days of administration, enabling the visualization of lesions as small as 1 cm in diameter. Notably, colon cancer studies demonstrated specific localization of tumors and metastatic lesions. However, SPECT/CT results revealed slow kidney clearance, necessitating 3–5 days to achieve optimal imaging outcomes. This delayed renal clearance has implications for the imaging capabilities of 131I-mAbF19 (Figure 1) [109].

2.2. Iodine-131-Labeled Sibrotuzumab
mAbF19 stands out for its FAP-specific targeting capabilities, and scientists have endeavored to enhance its imaging potential as a radiopharmaceutical [115]. A study by Welt et al. established the positive expression of FAP in all 17 patients studied, and SPECT/CT imaging effectively facilitated precise tumor identification in humans [109]. This encouraged further investigation into the pharmacokinetic (PK) of sibrotuzumab, a humanized version of the murine anti-FAP mAbF19. A phase I and II clinical trial (NCT02198274) involving sibrotuzumab sought to assess its PK without radiolabeling in patients with FAP-positive malignancies, particularly advanced metastatic colorectal cancer patients. However, among the 17 patients enrolled who had undergone rigorous pretreatment, only two exhibited stable disease status, a count insufficient to meet the phase II trial criteria, which typically necessitate four patients in a stable condition or at least one patient in complete or partial remission. Consequently, the trial did not progress beyond phase II [116]. The PK study unveiled pertinent findings, including a mean clearance rate of 39.8 ± 19.8 mL/h and a terminal half-life of 5.3 ± 2.3 days for sibrotuzumab.
One such avenue of exploration encompassed a clinical phase I dose-escalation study of sibrotuzumab in patients with colorectal or non-small cell lung cancer (NCT02209727). This study, conducted in parallel with the aforementioned phase I/II trials, sought to evaluate biodistribution, PKs, immunogenicity, and safety profiles by incrementally administering sibrotuzumab intravenously to 26 patients. In tandem, two antecedent phase I studies employed 131I-mAbF19, with a focus on patients with hepatic metastases from colorectal cancer or soft tissue sarcoma [117]. These investigations delved into the PK parameters of the therapeutic mouse monoclonal antibody 131I-mAbF19, elucidating principles of selective tumor accumulation and stromal targeting in tumors through biodistribution imaging studies and biopsy analyses. These pivotal insights provided the foundation for the inaugural human clinical evaluation of sibrotuzumab.
In this human trial, patients received 8–10 mCi of 131I-labeled sibrotuzumab, administered concomitantly at weeks 1, 5, and 9 in a 12-week dosing cycle, with a focus on PK assessments. The ensuing analysis divulged that the mean clearance rate of 131I-sibrotuzumab amounted to 41.9 ± 16 mL/h, accompanied by a half-life of 4.9 days. Following a single cycle of 131I-sibrotuzumab treatment, two out of the 26 patients manifested stable disease conditions. The relatively abbreviated half-life of sibrotuzumab held promise for radioimmunotherapy. Regrettably, the trial did not yield definitive efficacy results for sibrotuzumab, prompting the discontinuation of further clinical development (Figure 1) [113].
2.3. 177Lu-ESC11/ESC14
The clinical application of 131I-mAbF19 and its humanized derivative, sibrotuzumab, has been hindered by their prolonged blood clearance and suboptimal therapeutic efficacy. To enhance the therapeutic potential of FAP-targeted antibodies, it is imperative to develop antibodies with enhanced attributes and utilize radiolabeling with more suitable radionuclides [118][119][120][121].
From the perspective of antibody discovery, ESC11 and ESC14, two antibodies noted for their selective accumulation within xenografted FAP-positive human melanoma and their capacity to impede tumor growth in vivo, were identified in the human FAP antibody library using the phage display technique. Subsequently, these antibodies underwent a transformation into IgG1 antibodies [110]. The phage display technique is a new technique predicated on specific affinity interactions, enabling the identification of proteins or peptides that exhibit particular binding properties. This technique is especially adept at discovering antibodies targeting challenging and intriguing molecules, making it a cornerstone in the quest for antibodies with precise attributes [122][123][124][125][126]. It enjoys widespread utilization in the quest for human antibody fragments boasting specific binding activity [126][127][128]. This technique facilitates the evolution and optimization of FAP-targeting antibodies, culminating in the selection of antibodies exhibiting robust affinity, rapid internalization, and propensity for tumor accumulation.
From a radionuclide perspective, radiolabeling with iodine-131 is relatively straightforward for mAbs [129]. However, in the context of radioimmunotherapy, iodine-131 proves to be suboptimal due to its propensity for facile release from tumor sites after internalization of mAbs within cells [130][131]. A related drawback lies in the emission of high-energy (364 keV) γ-photons, accounting for a substantial 82% of its radiation output, thus posing concerns for radiation safety [132][133]. In stark contrast, the radioactive lanthanide 177Lu presents a more favorable profile, with a shorter emission range of 2 mm as opposed to iodine-131’s 3 mm.
These radiolabeled antibodies were subsequently administered to mice harboring SK-Mel-187 and SK-Mel-16 xenograft tumors to assess their tumor uptake. In this investigation, 177Lu-CHX-A″-DTPA-vF19 and 177Lu-CHX-A″-DTPA-A33 were included as control groups. Notably, SPECT/CT imaging conducted 72 h post-injection revealed a higher specific uptake of 177Lu-ESC11 in SK-MEL-187 tumors, whereas SK-MEL-16 xenografts exhibited lower uptake compared with the control group. This study, involving a comparative analysis of the in vivo targeting attributes of human–mouse chimeric antibodies, established that in mouse models characterized by higher levels of antigen expression, the cumulative tumor uptake of the nuclide-labeled antibody could reach levels corresponding to 50% of the administered dose per gram. Conclusive in vivo experiments in mice further corroborated that the ratio of tumor-to-organ uptake pertaining to 177Lu-labeled FAP mAbs ESC11 and ESC14 surpassed that of their first-generation radionuclide-labeled FAP-targeted antibodies [110]. The novel antibodies, ESC11 and ESC14, exhibited efficient internalization into FAP-expressing cells, thereby manifesting highly satisfactory in vivo targeting capabilities.
2.4. 89Zr-Labeled F19 and B12 IgG
Clinical investigations involving first-generation FAP-targeting antibodies have provided novel insights by demonstrating the feasibility of modifying FAP-specific cancer targeting through the conjugation of toxins or chelators with FAP-specific antibodies [134][135]. As an illustrative example, Pandya et al. prepared the radiopharmaceutical antibody conjugate [89Zr]Zr-Df-Bz-F19 mAb for PET imaging by employing the bifunctional chelator Df-Bz-NCS to securely bind zirconium-89 (89Zr) (Figure 1) [111].
89Zr, a radionuclide, emits β+ particles at 902 keV with an abundance of 23% and possesses a half-life of 78.4 h [132][136]. Its attributes, characterized by high-resolution imaging, specific tissue binding, and strong signal contrast, render it a promising candidate for PET imaging applications [137][138]. Nonetheless, certain challenges persist, primarily the susceptibility to covalent bond breakage between the chelator and the protein, which can compromise stability [139]. To mitigate this concern, comprehensive in vitro characterization of [89Zr]Zr-Df-Bz-F19 mAb was conducted. The radiolabel displayed a remarkable radioactive purity exceeding 99.5% upon synthesis completion and retained its purity at levels greater than 99.1% in human serum after 7 days of incubation at 36–37 °C. These findings affirm the robust stability of nucleoporin labeling and underscore the ability of [89Zr]Zr-Df-Bz-F19 to maintain its structural integrity following in vivo administration.
FAP expression is documented in multiple solid cancers, yet limited knowledge exists regarding its prevalence in metastatic castration-resistant prostate cancer (mCRPC) [140][141][142][143]. The evolving landscape of precision treatment strategies for prostate cancer was highlighted in the European Society of Medical Oncology 2022 report [144][145]. Advancements in CRPC therapies hinge on accurate imaging modalities, lesion visualization, disease staging, and informed therapeutic decision-making [132]. These findings underscore the ongoing demand for selective and sensitive imaging probes applicable to mCRPC patients.
To address the specific context of mCRPC, Hallie et al. employed a humanized antibody, initially identified by phage display, and labeled it with 89Zr. This antibody serves as a FAP-expressing tumor-selective imaging probe for PET/CT imaging in a preclinical prostate cancer xenograft model [112]. The investigative process commenced with genomic and immunohistochemistry assessments to determine the expression of FAP in prostate cancer [112][146][147]. Specifically, FAP localization in tissues and cells was determined via antigen–antibody binding reactions. Genomic analysis was predicated on RNA sequencing derived from primary prostate cancer patient samples or mCRPC bone and soft tissue tumor biopsies [148][149].
Subsequent PET/CT imaging evaluations were conducted in a mouse model established through the subcutaneous injection of CWR-R1FAP cells. Notably, [89Zr]Zr-B12 IgG demonstrated significantly enhanced tumor uptake in FAP-positive cells in mice bearing CWR-R1FAP relative to the control group injected with [89Zr]Zr-IC IgG. This heightened tumor accumulation is attributed to improved permeability and retention efficiency, leading to a sustained presence of the [89Zr]Zr-IC IgG probe within the tumor tissue, unlike the control probe, which exhibited rapid clearance and near-invisibility at 72 h. Compared with that in the control group, there was a heightened accumulation of [89Zr]Zr-B12 IgG in FAP-positive tumor cells (Figure 1) [112].
In a critical extension of this work, mice were subcutaneously injected with hPrCSC-44 (an immortalized human prostate cancer stromal cell line) in combination with DU145 (a FAP-null prostate cancer cell line) to establish a xenograft mouse model. Subsequent administration of [89Zr]Zr-B12 IgG or control [89Zr]Zr-IC IgG in mice bearing subcutaneous hPrCSC-44/DU145 xenografts, followed by serial imaging was performed at 24, 48, 72, 96, 120, and 144 h post-injection, allowed for comprehensive PET imaging evaluation. The results showed maximum tumor uptake of [89Zr]Zr-B12 IgG at 24 h, which surpassed control levels by 4–5 times.
2.5. Bispecific Antibodies
In the realm of tumor-targeting antibodies, an exciting development involves Hoffmann LaRoche’s bispecific antibodies RG7386 (FAP-DR5) and RO7300490 (FAP-CD40) (Figure 1) [114][150][151]. Currently in phase I clinical trials (NCT02558140, NCT04857138) [152][153], these antibodies represent a novel approach. One of these antibodies is engineered to target FAP for precise localization, while the other is designed to interact with molecules influencing tumor apoptosis or necrosis. These bispecific antibodies work in tandem, obstructing different signaling pathways simultaneously. Compared with monoclonal antibodies, bispecific antibodies possess two specific antigen-binding sites, which endow them with stronger specificity. Consequently, they precisely target tumor cells, minimize off-target toxicity, and can orchestrate immune cell-mediated tumor eradication through dual-target signal blockade [154][155][156][157][158][159][160][161]. This special structure confers a unique advantage to bispecific antibodies in the field of tumor therapy.
The concept of bispecific antibodies also opens up new possibilities for radionuclide labeling. While no specific studies have explored the radionuclide labeling of bispecific antibodies targeting FAP, research into combination therapies involving radionuclides and bispecific antibodies targeting other antigens has been conducted [162][163]. For instance, Morris’ team combined radionuclides with bispecific antibodies (anti-CTLA-4 and anti-PD-L1). In a mouse model, this approach resulted in complete and enduring tumor remission, outperforming combinations involving monoclonal antibodies [164]. The potential to combine or label FAP-targeted bispecific antibodies is a promising avenue that introduces a fresh dimension to radiotherapy nuclide markers for FAP-targeted tumor therapy. Such innovations hold significant potential for advancing clinical FAP-targeted tumor therapy.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics16030345
References
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840.
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68.
- Dart, A. Tumour microenvironment: Radical changes. Nat. Rev. Cancer 2018, 18, 65.
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59.
- Katheder, N.S.; Khezri, R.; O’Farrell, F.; Schultz, S.W.; Jain, A.; Rahman, M.M.; Schink, K.O.; Theodossiou, T.A.; Johansen, T.; Juhasz, G.; et al. Microenvironmental autophagy promotes tumour growth. Nature 2017, 541, 417–420.
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70–92.
- Gordon-Weeks, A.; Yuzhalin, A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331.
- Oudin, M.J.; Jonas, O.; Kosciuk, T.; Broye, L.C.; Guido, B.C.; Wyckoff, J.; Riquelme, D.; Lamar, J.M.; Asokan, S.B.; Whittaker, C.; et al. Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression. Cancer Discov. 2016, 6, 516–531.
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120.
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153–176.
- Berg, T.J.; Pietras, A. Radiotherapy-induced remodeling of the tumor microenvironment by stromal cells. Semin. Cancer Biol. 2022, 86, 846–856.
- Vitale, I.; Manic, G.; Galassi, C.; Galluzzi, L. Stress responses in stromal cells and tumor homeostasis. Pharmacol. Ther. 2019, 200, 55–68.
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84.
- Xie, Y.; Xie, F.; Zhang, L.; Zhou, X.; Huang, J.; Wang, F.; Jin, J.; Zhang, L.; Zeng, L.; Zhou, F. Targeted Anti-Tumor Immunotherapy Using Tumor Infiltrating Cells. Adv. Sci. 2021, 8, e2101672.
- Melssen, M.M.; Sheybani, N.D.; Leick, K.M.; Slingluff, C.L., Jr. Barriers to immune cell infiltration in tumors. J. Immunother. Cancer 2023, 11, e006401.
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205–226.
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912.
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45.
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166–181.
- Jiang, J.; Mei, J.; Ma, Y.; Jiang, S.; Zhang, J.; Yi, S.; Feng, C.; Liu, Y.; Liu, Y. Tumor hijacks macrophages and microbiota through extracellular vesicles. Exploration 2022, 2, 20210144.
- Liu, H.; Sun, B.; Zhu, P.; Liu, C.; Zhang, G.; Wang, D.; Song, X.; Shi, J.; Yang, Y.; Lu, J. Preparation of Three-Dimensional Porous Graphene by Hydrothermal and Chemical Reduction with Ascorbic Acid and its Electrochemical Properties. ChemistryOpen 2022, 11, e202200161.
- Zhang, D.X.; Vu, L.T.; Ismail, N.N.; Le, M.T.N.; Grimson, A. Landscape of extracellular vesicles in the tumour microenvironment: Interactions with stromal cells and with non-cell components, and impacts on metabolic reprogramming, horizontal transfer of neoplastic traits, and the emergence of therapeutic resistance. Semin. Cancer Biol. 2021, 74, 24–44.
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675.
- Arima, Y.; Matsueda, S.; Saya, H. Significance of Cancer-Associated Fibroblasts in the Interactions of Cancer Cells with the Tumor Microenvironment of Heterogeneous Tumor Tissue. Cancers 2023, 15, 2536.
- Li, Z.; Low, V.; Luga, V.; Sun, J.; Earlie, E.; Parang, B.; Shobana Ganesh, K.; Cho, S.; Endress, J.; Schild, T.; et al. Tumor-produced and aging-associated oncometabolite methylmalonic acid promotes cancer-associated fibroblast activation to drive metastatic progression. Nat. Commun. 2022, 13, 6239.
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163.
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598.
- Coller, H.A. Fibroblasts Prompt Tumors to Mobilize Their Glycogen Reserves. Trends Cell Biol. 2019, 29, 278–280.
- Cully, M. Tumour microenvironment: Fibroblast subtype provides niche for cancer stem cells. Nat. Rev. Cancer 2018, 18, 136.
- Roulis, M.; Kaklamanos, A.; Schernthanner, M.; Bielecki, P.; Zhao, J.; Kaffe, E.; Frommelt, L.S.; Qu, R.; Knapp, M.S.; Henriques, A.; et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 2020, 580, 524–529.
- Park, D.; Sahai, E.; Rullan, A. SnapShot: Cancer-Associated Fibroblasts. Cell 2020, 181, 486.e481.
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115.
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807.
- Prakash, J. Cancer-Associated Fibroblasts: Perspectives in Cancer Therapy. Trends Cancer 2016, 2, 277–279.
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. Landmark Ed. 2010, 15, 166–179.
- Alvarez-Teijeiro, S.; Garcia-Inclan, C.; Villaronga, M.A.; Casado, P.; Hermida-Prado, F.; Granda-Diaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Rio-Ibisate, N.; Gandarillas, A.; et al. Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets. Cancers 2018, 10, 334.
- Piper, M.; Mueller, A.C.; Karam, S.D. The interplay between cancer associated fibroblasts and immune cells in the context of radiation therapy. Mol. Carcinog. 2020, 59, 754–765.
- Allam, A.; Yakou, M.; Pang, L.; Ernst, M.; Huynh, J. Exploiting the STAT3 Nexus in Cancer-Associated Fibroblasts to Improve Cancer Therapy. Front. Immunol. 2021, 12, 767939.
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131.
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176.
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218–252.
- Hausmann, C.; Zoschke, C.; Wolff, C.; Darvin, M.E.; Sochorova, M.; Kovacik, A.; Wanjiku, B.; Schumacher, F.; Tigges, J.; Kleuser, B.; et al. Fibroblast origin shapes tissue homeostasis, epidermal differentiation, and drug uptake. Sci. Rep. 2019, 9, 2913.
- Mueller, L.; Goumas, F.A.; Affeldt, M.; Sandtner, S.; Gehling, U.M.; Brilloff, S.; Walter, J.; Karnatz, N.; Lamszus, K.; Rogiers, X.; et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am. J. Pathol. 2007, 171, 1608–1618.
- Li, A.; Chen, Y.S.; Ping, X.L.; Yang, X.; Xiao, W.; Yang, Y.; Sun, H.Y.; Zhu, Q.; Baidya, P.; Wang, X.; et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017, 27, 444–447.
- Sebastian, A.; Hum, N.R.; Martin, K.A.; Gilmore, S.F.; Peran, I.; Byers, S.W.; Wheeler, E.K.; Coleman, M.A.; Loots, G.G. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers 2020, 12, 1307.
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925.
- Shook, B.A.; Wasko, R.R.; Rivera-Gonzalez, G.C.; Salazar-Gatzimas, E.; Lopez-Giraldez, F.; Dash, B.C.; Munoz-Rojas, A.R.; Aultman, K.D.; Zwick, R.K.; Lei, V.; et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018, 362, eaar2971.
- Yu, J.; Seldin, M.M.; Fu, K.; Li, S.; Lam, L.; Wang, P.; Wang, Y.; Huang, D.; Nguyen, T.L.; Wei, B.; et al. Topological Arrangement of Cardiac Fibroblasts Regulates Cellular Plasticity. Circ. Res. 2018, 123, 73–85.
- Jiang, D.; Rinkevich, Y. Converting fibroblastic fates leads to wound healing without scar. Signal Transduct. Target. Ther. 2021, 6, 332–334.
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120.
- Hutchenreuther, J.; Leask, A. A tale of two orgins: Do myofibroblasts originate from different sources in wound healing and fibrosis? Cell Tissue Res. 2016, 365, 507–509.
- Foster, D.S.; Januszyk, M.; Yost, K.E.; Chinta, M.S.; Gulati, G.S.; Nguyen, A.T.; Burcham, A.R.; Salhotra, A.; Ransom, R.C.; Henn, D.; et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc. Natl. Acad. Sci. USA 2021, 118, e2110025118.
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659.
- Byun, J.S.; Gardner, K. Wounds that will not heal: Pervasive cellular reprogramming in cancer. Am. J. Pathol. 2013, 182, 1055–1064.
- Koustoulidou, S.; Hoorens, M.W.H.; Dalm, S.U.; Mahajan, S.; Debets, R.; Seimbille, Y.; de Jong, M. Cancer-Associated Fibroblasts as Players in Cancer Development and Progression and Their Role in Targeted Radionuclide Imaging and Therapy. Cancers 2021, 13, 1100.
- Hosein, A.N.; Wu, M.; Arcand, S.L.; Lavallee, S.; Hebert, J.; Tonin, P.N.; Basik, M. Breast carcinoma-associated fibroblasts rarely contain p53 mutations or chromosomal aberrations. Cancer Res. 2010, 70, 5770–5777.
- Zhai, X.; Chen, X.; Wan, Z.; Ge, M.; Ding, Y.; Gu, J.; Hua, J.; Guo, D.; Tan, M.; Xu, D. Identification of the novel therapeutic targets and biomarkers associated of prostate cancer with cancer-associated fibroblasts (CAFs). Front. Oncol. 2023, 13, 1136835.
- van der Heide, C.D.; Dalm, S.U. Radionuclide imaging and therapy directed towards the tumor microenvironment: A multi-cancer approach for personalized medicine. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4616–4641.
- Imlimthan, S.; Moon, E.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023.
- Robb, M.A.; McInnes, P.M.; Califf, R.M. Biomarkers and Surrogate Endpoints. JAMA 2016, 315, 1107–1108.
- Liu, D. Cancer biomarkers for targeted therapy. Biomark. Res. 2019, 7, 25.
- Zhang, J.; Gu, C.; Song, Q.; Zhu, M.; Xu, Y.; Xiao, M.; Zheng, W. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020, 10, 127.
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434.
- Bai, J.W.; Qiu, S.Q.; Zhang, G.J. Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduct. Target. Ther. 2023, 8, 89–120.
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463.
- Brennen, W.N.; Rosen, D.M.; Wang, H.; Isaacs, J.T.; Denmeade, S.R. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl. Cancer Inst. 2012, 104, 1320–1334.
- Choyke, P.L. PET of Fibroblast-Activation Protein for Cancer Staging: What We Know and What We Need to Learn. Radiology 2022, 304, 658–659.
- Han, C.; Liu, T.; Yin, R. Biomarkers for cancer-associated fibroblasts. Biomark. Res. 2020, 8, 64.
- Mayola, M.F.; Thackeray, J.T. The Potential of Fibroblast Activation Protein-Targeted Imaging as a Biomarker of Cardiac Remodeling and Injury. Curr. Cardiol. Rep. 2023, 25, 515–523.
- Ebert, L.M.; Yu, W.; Gargett, T.; Toubia, J.; Kollis, P.M.; Tea, M.N.; Ebert, B.W.; Bardy, C.; van den Hurk, M.; Bonder, C.S.; et al. Endothelial, pericyte and tumor cell expression in glioblastoma identifies fibroblast activation protein (FAP) as an excellent target for immunotherapy. Clin. Transl. Immunol. 2020, 9, e1191.
- Rezaei, S.; Gharapapagh, E.; Dabiri, S.; Heidari, P.; Aghanejad, A. Theranostics in targeting fibroblast activation protein bearing cells: Progress and challenges. Life Sci. 2023, 329, 121970.
- Levy, M.T.; McCaughan, G.W.; Abbott, C.A.; Park, J.E.; Cunningham, A.M.; Muller, E.; Rettig, W.J.; Gorrell, M.D. Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 1999, 29, 1768–1778.
- Juillerat-Jeanneret, L.; Tafelmeyer, P.; Golshayan, D. Fibroblast activation protein-alpha in fibrogenic disorders and cancer: More than a prolyl-specific peptidase? Expert Opin. Ther. Targets 2017, 21, 977–991.
- Zhang, T.; Tong, X.; Zhang, S.; Wang, D.; Wang, L.; Wang, Q.; Fan, H. The Roles of Dipeptidyl Peptidase 4 (DPP4) and DPP4 Inhibitors in Different Lung Diseases: New Evidence. Front. Pharmacol. 2021, 12, 731453.
- Han, R.; Wang, X.; Bachovchin, W.; Zukowska, Z.; Osborn, J.W. Inhibition of dipeptidyl peptidase 8/9 impairs preadipocyte differentiation. Sci. Rep. 2015, 5, 12348.
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012, 11, 257–266.
- Zubal, M.; Vymolova, B.; Matrasova, I.; Vymola, P.; Veprkova, J.; Syrucek, M.; Tomas, R.; Vanickova, Z.; Krepela, E.; Konecna, D.; et al. Fibroblast activation protein as a potential theranostic target in brain metastases of diverse solid tumours. Pathology 2023, 55, 806–817.
- Wonganu, B.; Berger, B.W. A specific, transmembrane interface regulates fibroblast activation protein (FAP) homodimerization, trafficking and exopeptidase activity. Biochim. Biophys. Acta 2016, 1858, 1876–1882.
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946.
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512.
- Kalaei, Z.; Manafi-Farid, R.; Rashidi, B.; Kiani, F.K.; Zarei, A.; Fathi, M.; Jadidi-Niaragh, F. The Prognostic and therapeutic value and clinical implications of fibroblast activation protein-alpha as a novel biomarker in colorectal cancer. Cell Commun. Signal 2023, 21, 139.
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J. Biol. Chem. 2005, 280, 19441–19444.
- Niedermeyer, J.; Garin-Chesa, P.; Kriz, M. Expression of the fibroblast activation protein during mouse embryo development. Int. J. Dev. Biol. 2001, 45, 445–447.
- Niedermeyer, J.; Kriz, M.; Hilberg, F.; Garin-Chesa, P.; Bamberger, U.; Lenter, M.C.; Park, J.; Viertel, B.; Püschner, H.; Mauz, M.; et al. Targeted Disruption of Mouse Fibroblast Activation Protein. Mol. Cell Biol. 2000, 20, 1089–1094.
- Brown, D.D.; Wang, Z.; Furlow, J.D.; Kanamori, A. The thyroid hormone-induced tail resorption program during Xenopus laevis metamorphosis. Proc. Natl. Acad. Sci. USA 1996, 93, 1924–1929.
- Rettig, W.J.; Su, S.L.; Fortunato, S.R.; Scanlan, M.J.; Raj, B.K.; Garin-Chesa, P.; Healey, J.H.; Old, L.J. Fibroblast activation protein: Purification, epitope mapping and induction by growth factors. Int. J. Cancer 1994, 58, 385–392.
- Dolznig, H.; Schweifer, N.; Puri, C. Characterization of cancer stroma markers: In silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer Immun. 2005, 5, 10.
- Egger, C.; Cannet, C.; Gerard, C.; Suply, T.; Ksiazek, I.; Jarman, E.; Beckmann, N. Effects of the fibroblast activation protein inhibitor, PT100, in a murine model of pulmonary fibrosis. Eur. J. Pharmacol. 2017, 809, 64–72.
- Tillmanns, J.; Hoffmann, D.; Habbaba, Y.; Schmitto, J.D.; Sedding, D.; Fraccarollo, D.; Galuppo, P.; Bauersachs, J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J. Mol. Cell Cardiol. 2015, 87, 194–203.
- Uitte de Willige, S.; Malfliet, J.J.; Janssen, H.L.; Leebeek, F.W.; Rijken, D.C. Increased N-terminal cleavage of alpha-2-antiplasmin in patients with liver cirrhosis. J. Thromb. Haemost. 2013, 11, 2029–2036.
- Nagaraju, C.K.; Dries, E.; Popovic, N.; Singh, A.A.; Haemers, P.; Roderick, H.L.; Claus, P.; Sipido, K.R.; Driesen, R.B. Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction. Sci. Rep. 2017, 7, 10801–10815.
- Rettig, W.; Garin-Chesa, P.; Beresford, H.; Oettegen, H. Cell-surface glycoproteins of human sarcomas: Differential expression in normal and malignant tissues and cultured cells. Proc. Natl. Acad. Sci. USA 1988, 85, 3110–3114.
- Bauer, S.; Jendro, M.C.; Wadle, A.; Kleber, S.; Stenner, F.; Dinser, R.; Reich, A.; Faccin, E.; Godde, S.; Dinges, H.; et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res. Ther. 2006, 8, R171–R182.
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239.
- Mentlein, R.; Hattermann, K.; Hemion, C.; Jungbluth, A.A.; Held-Feindt, J. Expression and role of the cell surface protease seprase/fibroblast activation protein-alpha (FAP-alpha) in astroglial tumors. Biol. Chem. 2011, 392, 199–207.
- Brokopp, C.E.; Schoenauer, R.; Richards, P.; Bauer, S.; Lohmann, C.; Emmert, M.Y.; Weber, B.; Winnik, S.; Aikawa, E.; Graves, K.; et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur. Heart J. 2011, 32, 2713–2722.
- Wang, X.M.; Yu, D.M.; McCaughan, G.W.; Gorrell, M.D. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology 2005, 42, 935–945.
- Scanlan, M.J.; Raj, B.K.; Calvo, B.; Garin-Chesa, P.; Sanz-Moncasi, M.P.; Healey, J.H.; Old, L.J.; Rettig, W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661.
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658.
- Rettig, W.J.; Chesa, P.G.; Beresford, H.R.; Feickert, H.J.; Jennings, M.T.; Cohen, J.; Oettgen, H.F.; Old, L.J. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res. 1986, 46, 6406–6412.
- Rettig, W.J.; Garin-Chesa, P.; Healey, J.H.; Su, S.L.; Ozer, H.L.; Schwab, M.; Albino, A.P.; Old, L.J. Regulation and Heteromeric Structure of the Fibroblast Activation Protein in Normal and Transformed Cells of Mesenchymal and Neuroectodermal Origin1. Cancer Res. 1993, 53, 3327–3335.
- O’Brien, P.; O’Connor, B.F. Seprase: An overview of an important matrix serine protease. Biochim. Biophys. Acta 2008, 1784, 1130–1145.
- Lo, P.-C.; Chen, J.; Stefflova, K.; Warren, M.S.; Navab, R.; Bandarchi, B.; Mullins, S.; Tsao, M.; Cheng, J.D.; Zheng, G. Photodynamic Molecular Beacon Triggered by Fibroblast Activation Protein on Cancer-Associated Fibroblasts for Diagnosis and Treatment of Epithelial Cancers. J. Med. Chem. 2009, 52, 358–368.
- Peltier, A.; Seban, R.D.; Buvat, I.; Bidard, F.C.; Mechta-Grigoriou, F. Fibroblast heterogeneity in solid tumors: From single cell analysis to whole-body imaging. Semin. Cancer Biol. 2022, 86, 262–272.
- Kelly, T. Fibroblast activation protein-alpha and dipeptidyl peptidase IV (CD26): Cell-surface proteases that activate cell signaling and are potential targets for cancer therapy. Drug Resist. Updat. 2005, 8, 51–58.
- Prive, B.M.; Boussihmad, M.A.; Timmermans, B.; van Gemert, W.A.; Peters, S.M.B.; Derks, Y.H.W.; van Lith, S.A.M.; Mehra, N.; Nagarajah, J.; Heskamp, S.; et al. Fibroblast activation protein-targeted radionuclide therapy: Background, opportunities, and challenges of first (pre)clinical studies. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1906–1918.
- Jung, H.J.; Nam, E.H.; Park, J.Y.; Ghosh, P.; Kim, I.S. Identification of BR102910 as a selective fibroblast activation protein (FAP) inhibitor. Bioorg. Med. Chem. Lett. 2021, 37, 127846.
- Aoyama, A.; Chen, W.-T. A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc. Natl. Acad. Sci. USA 1990, 87, 8296–8300.
- Welt, S.; Divgi, C.R.; Scott, A.M.; Garin-Chesa, P.; Finn, R.D.; Graham, M.; Carswell, E.A.; Cohen, A.; Larson, S.M.; Old, L.J.; et al. Antibody Targeting in Metastatic Colon Cancer: A Phase I Study of Monoclonal Antibody F19 Against a Cell-Surface Protein of Reactive Tumor Stromal Fibroblasts. J. Clin. Oncol. 1994, 12, 1193–1203.
- Fischer, E.; Chaitanya, K.; Wuest, T.; Wadle, A.; Scott, A.M.; van den Broek, M.; Schibli, R.; Bauer, S.; Renner, C. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin. Cancer Res. 2012, 18, 6208–6218.
- Pandya, D.N.; Sinha, A.; Yuan, H.; Mutkus, L.; Stumpf, K.; Marini, F.C.; Wadas, T.J. Imaging of Fibroblast Activation Protein Alpha Expression in a Preclinical Mouse Model of Glioma Using Positron Emission Tomography. Molecules 2020, 25, 3672.
- Hintz, H.M.; Gallant, J.P.; Vander Griend, D.J.; Coleman, I.M.; Nelson, P.S.; LeBeau, A.M. Imaging Fibroblast Activation Protein Alpha Improves Diagnosis of Metastatic Prostate Cancer with Positron Emission Tomography. Clin. Cancer Res. 2020, 26, 4882–4891.
- Scott, A.M.; Wiseman, G.; Welt, S.; Adjei, A.; Lee, F.T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 2003, 9, 1639–1647.
- Brunker, P.; Wartha, K.; Friess, T.; Grau-Richards, S.; Waldhauer, I.; Koller, C.F.; Weiser, B.; Majety, M.; Runza, V.; Niu, H.; et al. RG7386, a Novel Tetravalent FAP-DR5 Antibody, Effectively Triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and Tumor Cell Apoptosis. Mol. Cancer Ther. 2016, 15, 946–957.
- Kim, E.E. Therapeutic Nuclear Medicine. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 163.
- Hofheinz, R.D.; al-Batran, S.E.; Hartmann, F.; Hartung, G.; Jäger, D.; Renner, C.; Tanswell, P.; Kunz, U.; Amelsberg, A.; Kuthan, H.; et al. Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Oncol. Res. Treat. 2003, 26, 44–48.
- Tanswell, P.; Garin-Chesa, P.; Rettig, W.J.; Welt, S.; Divgi, C.R.; Casper, E.S.; Finn, R.D.; Larson, S.M.; Old, L.J.; Scott, A.M. Population pharmacokinetics of antifibroblast activation protein monoclonal antibody F19 in cancer patients. Br. J. Clin. Pharmacol. 2001, 51, 177–180.
- Ingelheim, B. Single Dose Escalation Study of 131I-Sibrotuzumab in Patients with Advanced or Metastatic Non-small Cell Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT02209727 (accessed on 6 August 2014).
- Starr, C.G.; Tessier, P.M. Selecting and engineering monoclonal antibodies with drug-like specificity. Curr. Opin. Biotechnol. 2019, 60, 119–127.
- Chatal, J.-F.; Hoefnagel, C.A. Radionuclide therapy. Lancet 1999, 354, 931–935.
- DeNardo, S.J.; DeNardo, G.L. Targeted radionuclide therapy for solid tumors: An overview. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, S89–S95.
- Ledsgaard, L.; Ljungars, A.; Rimbault, C.; Sorensen, C.V.; Tulika, T.; Wade, J.; Wouters, Y.; McCafferty, J.; Laustsen, A.H. Advances in antibody phage display technology. Drug Discov. Today 2022, 27, 2151–2169.
- Mimmi, S.; Maisano, D.; Quinto, I.; Iaccino, E. Phage Display: An Overview in Context to Drug Discovery. Trends Pharmacol. Sci. 2019, 40, 87–91.
- Saw, P.E.; Song, E.-W. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 2019, 10, 787–807.
- Schofield, D.J.; Pope, A.R.; Clementel, V.; Buckell, J.; Chapple, S.D.J.; Clarke, K.F.; Conquer, J.S.; Crofts, A.M.; Crowther, S.R.E.; Dyson, M.R.; et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol. 2007, 8, R254.
- Frenzel, A.; Kügler, J.; Helmsing, S.; Meier, D.; Schirrmann, T.; Hust, M.; Dübel, S. Designing Human Antibodies by Phage Display. Transfus. Med. Hemother. 2017, 44, 312–318.
- Nagano, K.; Tsutsumi, Y. Phage Display Technology as a Powerful Platform for Antibody Drug Discovery. Viruses 2021, 13, 178.
- Peissert, F.; Plüss, L.; Giudice, A.M.; Ongaro, T.; Villa, A.; Elsayed, A.; Nadal, L.; Dakhel Plaza, S.; Scietti, L.; Puca, E.; et al. Selection of a PD-1 blocking antibody from a novel fully human phage display library. Protein Sci. 2022, 31, e4486.
- Gupta, S.; Batra, S.; Jain, M. Antibody Labeling with Radioiodine and Radiometals. Drug Deliv. Syst. 2014, 1141, 147–157.
- Stein, R.; Govindan, S.V.; Mattes, M.J.; Chen, S.; Reed, L.; Newsome, G.; McBride, B.J.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res. 2003, 63, 111–118.
- Anderson, W.T.; Strand, M. Radiolabeled antibody: Iodine versus radiometal chelates. NCI Monogr. 1987, 3, 149–151.
- Sun, J.; Huangfu, Z.; Yang, J.; Wang, G.; Hu, K.; Gao, M.; Zhong, Z. Imaging-guided targeted radionuclide tumor therapy: From concept to clinical translation. Adv. Drug Deliv. Rev. 2022, 190, 114538.
- Brouwers, A.H.; van Eerd, J.E.M.; Frielink, C.; Oosterwijk, E.; Oyen, W.J.G.; Corstens, F.H.M.; Boerman, O.C. Optimization of Radioimmunotherapy of Renal Cell Carcinoma: Labeling of Monoclonal Antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J. Nucl. Med. 2004, 45, 327–337.
- Hoffmann, R.M.; Mele, S.; Cheung, A.; Larcombe-Young, D.; Bucaite, G.; Sachouli, E.; Zlatareva, I.; Morad, H.O.J.; Marlow, R.; McDonnell, J.M.; et al. Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-Drug Conjugates (ADCs). Sci. Rep. 2020, 10, 8869.
- Morais, M.; Ma, M.T. Site-specific chelator-antibody conjugation for PET and SPECT imaging with radiometals. Drug Discov. Today Technol. 2018, 30, 91–104.
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8.
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with (8)(9)Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14.
- De Feo, M.S.; Pontico, M.; Frantellizzi, V.; Corica, F.; De Cristofaro, F.; De Vincentis, G. 89Zr-PET imaging in humans: A systematic review. Clin. Transl. Imaging 2021, 10, 23–36.
- Fischer, G.; Seibold, U.; Schirrmacher, R.; Wangler, B.; Wangler, C. 89Zr, a radiometal nuclide with high potential for molecular imaging with PET: Chemistry, applications and remaining challenges. Molecules 2013, 18, 6469–6490.
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090.
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, A.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142.
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804.
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474.
- oncologypro.esmo.org/tumour-sites. Available online: https://oncologypro.esmo.org/tumour-sites/genitourinary-cancers/prostate-cancer (accessed on 29 January 2024).
- oncologypro.esmo.org/meeting-resources. Available online: https://oncologypro.esmo.org/meeting-resources/molecular-analysis-for-precision-oncology-congress-2022/multi-focal-genomic-dissection-of-synchronous-primary-and-metastatic-tissue-from-de-novo-metastatic-prostate-cancer (accessed on 29 January 2024).
- Lappalainen, T.; Scott, A.J.; Brandt, M.; Hall, I.M. Genomic Analysis in the Age of Human Genome Sequencing. Cell 2019, 177, 70–84.
- De Smet, F.; Antoranz Martinez, A.; Bosisio, F.M. Next-Generation Pathology by Multiplexed Immunohistochemistry. Trends Biochem. Sci. 2021, 46, 80–82.
- Pflueger, D.; Terry, S.; Sboner, A.; Habegger, L.; Esgueva, R.; Lin, P.C.; Svensson, M.A.; Kitabayashi, N.; Moss, B.J.; MacDonald, T.Y.; et al. Discovery of non-ETS gene fusions in human prostate cancer using next-generation RNA sequencing. Genome Res. 2011, 21, 56–67.
- Bhanvadia, R.R.; VanOpstall, C.; Brechka, H.; Barashi, N.S.; Gillard, M.; McAuley, E.M.; Vasquez, J.M.; Paner, G.; Chan, W.C.; Andrade, J.; et al. MEIS1 and MEIS2 Expression and Prostate Cancer Progression: A Role For HOXB13 Binding Partners in Metastatic Disease. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3668–3680.
- Sum, E.; Rapp, M.; Frobel, P.; Le Clech, M.; Durr, H.; Giusti, A.M.; Perro, M.; Speziale, D.; Kunz, L.; Menietti, E.; et al. Fibroblast Activation Protein alpha-Targeted CD40 Agonism Abrogates Systemic Toxicity and Enables Administration of High Doses to Induce Effective Antitumor Immunity. Clin. Cancer Res. 2021, 27, 4036–4053.
- Labiano, S.; Roh, V.; Godfroid, C.; Hiou-Feige, A.; Romero, J.; Sum, E.; Rapp, M.; Boivin, G.; Wyss, T.; Simon, C.; et al. CD40 Agonist Targeted to Fibroblast Activation Protein alpha Synergizes with Radiotherapy in Murine HPV-Positive Head and Neck Tumors. Clin. Cancer Res. 2021, 27, 4054–4065.
- clinicaltrials.gov. NCT02558140. Available online: https://clinicaltrials.gov/study/NCT02558140 (accessed on 1 October 2022).
- clinicaltrials.gov. NCT04857138. Available online: https://clinicaltrials.gov/study/NCT04857138?cond=NCT04857138&rank=1 (accessed on 1 October 2022).
- Wang, F.; Tsai, J.C.; Davis, J.H.; Chau, B.; Dong, J.; West, S.M.; Hogan, J.M.; Wheeler, M.L.; Bee, C.; Morishige, W.; et al. Design and characterization of mouse IgG1 and IgG2a bispecific antibodies for use in syngeneic models. mAbs 2019, 12, 1685350.
- Meermeier, E.W.; Welsh, S.J.; Sharik, M.E.; Du, M.T.; Garbitt, V.M.; Riggs, D.L.; Shi, C.-X.; Stein, C.K.; Bergsagel, M.; Chau, B.; et al. Tumor Burden Limits Bispecific Antibody Efficacy through T-cell Exhaustion Averted by Concurrent Cytotoxic Therapy. Blood Cancer Discov. 2021, 2, 354–369.
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616.
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344.
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713.
- Hingorani, P.; Krailo, M.D.; Buxton, A.; Hutson, P.R.; Davis, J.; Janeway, K.A.; Gorlick, R.G.; Isakoff, M. Phase II study of antidisialoganglioside antibody, dinutuximab, in combination with GM-CSF in patients with recurrent osteosarcoma (AOST1421): A report from the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 10508.
- Edelman, M.; Dvorkin, M.; Laktionov, K.K.; Navarro, A.; Juan-Vidal, O.; Kozlov, V.; Golden, G.; Jordan, O.; Deng, C. The anti-disialoganglioside (GD2) antibody dinutuximab (D) for second-line treatment (2LT) of patients (pts) with relapsed/refractory small cell lung cancer (RR SCLC): Results from part II of the open-label, randomized, phase II/III distinct study. J. Clin. Oncol. 2020, 38, 9017.
- Sikic, B.I.; Narayanan, S.; Colevas, A.D.; Padda, S.K.; Fisher, G.A.; Supan, D.; Wakelee, H.A.; Aoki, R.; Pegram, M.D.; Villalobos, V.M.; et al. A first-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 2016, 34, 3019.
- Pharmaceuticals, F. Fusion Pharmaceuticals Announces IND Clearance for FPI-2068, A Jointly Developed Novel Targeted Alpha Therapy. 2023. Available online: https://ir.fusionpharma.com/2023-04-12-Fusion-Pharmaceuticals-Announces-IND-Clearance-for-FPI-2068,-a-Jointly-Developed-Novel-Targeted-Alpha-Therapy (accessed on 1 June 2023).
- Moores, S.L.; Chiu, M.L.; Bushey, B.S.; Chevalier, K.; Luistro, L.; Dorn, K.; Brezski, R.J.; Haytko, P.; Kelly, T.; Wu, S.-J.; et al. A Novel Bispecific Antibody Targeting EGFR and cMet Is Effective against EGFR Inhibitor–Resistant Lung Tumors. Cancer Res. 2016, 76, 3942–3953.
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabb3631.
This entry is offline, you can click here to edit this entry!
