Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Electrochemistry
Proton exchange membrane fuel cells (PEMFCs) have the potential to tackle major challenges associated with fossil fuel-sourced energy consumption. Nafion, a perfluorosulfonic acid (PFSA) membrane that has high proton conductivity and good chemical stability, is a standard proton exchange membrane (PEM) used in PEMFCs. However, PEM degradation is one of the significant issues in the long-term operation of PEMFCs. Membrane degradation can lead to a decrease in the performance and the lifespan of PEMFCs. The membrane can degrade through chemical, mechanical, and thermal pathways.
- proton exchange membrane (PEM)
- fuel cells
- thermal stability
- conductivity
- chemical
- composite
1. Introduction
Rapid population growth and industrialization requiring enormous amounts of fossil fuels-based energy have led to excessive emissions of greenhouse gases, mainly CO2, causing severe environmental issues. Recent environmental awareness and stringent governmental policies have forced the world to transition from quickly depleting and pollution-causing fossil fuel-based energy to green and alternative renewable sources of energy. Hence, researchers are exploring greener alternative sources of energy [1,2] and, at the same time, possible ways to utilize the CO2 emission streams [3,4,5]. There are several sources of energy, such as wind, solar, geothermal, and hydropower, that are greener and renewable. Nevertheless, the intermittent nature and unpredictability of these sources present significant challenges to ensuring their stable operation as reliable energy sources for end users. Fuel cells, which are electrochemical devices that can convert the chemical energy stored in a fuel into electrical energy without actual fuel combustion, could be a potential solution. Depending on the nature of the electrolyte used, fuel cells can be categorized as polymer electrolyte (proton exchange) membrane fuel cells (PEMFCs), phosphoric acid fuel cells (PAFCs), molten carbonate fuel cells (MCFCs), direct methanol fuel cells (DMFCs), alkaline fuel cells (AFCs), and solid oxide fuel cells (SOFCs) [6,7]. Proton exchange membrane fuel cells (PEMFCs), which convert the chemical energy of hydrogen that can be produced using renewable energy sources such as solar, wind, etc., into electrical energy, have the potential to tackle the issues originating from the intermittent and unpredictable nature of renewable sources [8]. Features such as high compactness, high power density, noiseless operation, and efficient energy production without releasing pollutants or CO2 make PEMFCs a promising cleaner energy source [9].
PEMFCs mainly consist of membrane–electrode assembly (MEA), bipolar plates, and sealing gaskets in a sandwich structure (Figure 1a). A fuel cell stack is formed by several MEAs, and each MEA is sandwiched between two bipolar plates, providing a foundation and mechanical support by acting as a shield to the fuel cell. Gaskets are added around the edges of the MEA to prevent any gas leaks. The electrochemical reaction takes place at the MEA, which is composed of an anode and a cathode, each containing a gas diffusion layer and an active catalyst layer separated by a proton exchange membrane (PEM). Hydrogen is injected as fuel on the anode side and diffuses through the gas diffusion layer to reach the anode catalyst layer. The catalyst facilitates hydrogen oxidization to produce protons (H+) and electrons (e−) (1). The proton exchange membrane allows protons to pass through to reach the cathode catalyst layer while electrons are transferred to the cathode via an external circuit. Air is injected on the cathode side and reaches the cathode catalyst layer, flowing through the gas diffusion layer. The cathodic catalyst enables oxygen reduction by reacting with the protons that have crossed the membrane and electrons available from the external circuit (2), producing water and heat using H2 and O2 in the overall process (3) (Figure 1b).
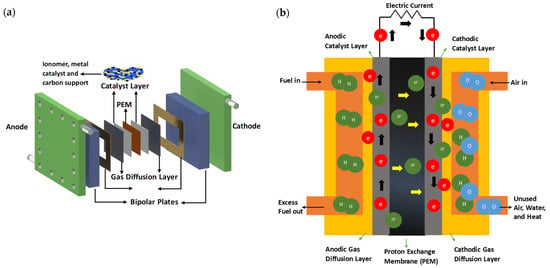
Figure 1. (a) Schematic showing the components of a PEMFC: bipolar plates, sealing gaskets, gas diffusion layers, catalyst layers, and the proton exchange membrane; this arrangement is repeated in a fuel cell stack. (b) Description of the operation of a PEMFC.
The proton exchange membrane is the key element of the PEMFC, as it must permit only the protons to pass while preventing direct electron conduction and gas permeation between the anode and cathode. Such a requirement makes it essential for PEM to have excellent proton conductivity and extremely low gas and electron permeability. Furthermore, PEMFCs are operated in harsh working conditions integrating water, air, hydrogen, and heat, necessitating the PEM to have sufficient chemical, mechanical, thermal, and dimensional stability [10]. Several polymers have been considered as PEMs for their application in PEMFCs under diverse operating conditions [11,12,13,14]. They can be mainly categorized as non-fluorinated and fluorinated polymeric membranes. The latter has been preferred because most non-fluorinated membranes, although having attractive prices, have poor resistance to oxidation and undergo thermal degradation. Fluorinated proton exchange membranes, especially fully fluorinated (perfluorinated) ones, have the advantageous combination of chemical, thermal, mechanical, and dimensional stability.
Nafion, a perfluorosulfonic acid membrane, was developed in the mid-1960s by DuPont and is the most used PEM in PEMFCs. It consists of an aliphatic perfluorocarbon hydrophobic chain and hydrophilic sulfonic acid terminated perfluoro vinyl ether side groups that exhibit excellent proton conductivity and good chemical, oxidative, and dimensional stability [15]. However, it has been found that PEM degradation is one of the significant issues in the long-term operation of PEMFCs. The membrane degradation can lead to a decrease in fuel cell performance and a shorter lifespan. While Nafion has been a dominant proton exchange membrane, several polymers with similar core structures, such as Aquivion® and Hyflon® (earlier DOW) by Solvay (Brussels, Belgium), Flemion® by Asahi Glass, (Chiyoda City, Tokyo, Japan) and DyneonTM by 3M (Saint Paul, MN, USA), etc. (Figure 2), have been studied as PEMs in fuel cells [16,17]. Given their similar core structures, the degradation issue is analogous to that of Nafion; therefore, the discussions in this research could be applied to all similar polymers.
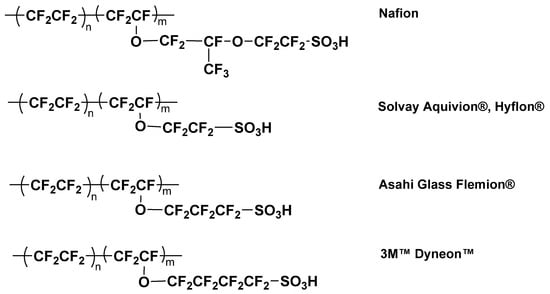
Figure 2. Chemical structures of some commercial perfluorosulfonic acid-based proton exchange membranes.
The advances in PEM fuel cell membrane development target improving thermal stability for high-temperature applications, along with enhanced conductivity and mechanical properties [118,119,120]. The approaches can be divided into (i) modifications in the chemistry of membrane materials and (ii) developing composite membranes.
2. Chemically Modified PEMs
Several attempts have been made to modify the structure of Nafion to achieve advantageous properties. The research has led to several commercial membranes, such as Aquivion®, Hyflon®, Flemion®, and DyneonTM, based on shorter side sulfonic chains compared with Nafion [119]. Furthermore, the whole range of new materials has been tested as proton exchange membranes for high-temperature fuel cell applications. Several sulfonated polymers, such as polyether ketones, polyphenylsulfone, and poly(arylene ether), have been widely studied as PEMs due to their good stability at high temperatures and low cost [121,122,123,124,125,126]. However, sulfonated polyether ketone membranes, like the PFSA membranes, undergo degradation due to dehydration under high operating temperatures. Polybenzimidazole (PBI) is an aromatic linear heterocyclic rigid semi-crystalline polymer containing repeated benzimidazole units with excellent chemical resistance, high mechanical strength, good moisture regain, and thermo-oxidative stability above 80 °C [127]. PBI and acid-doped PBI-based membranes have been considered for high-temperature applications because of their excellent chemical and thermal properties at elevated temperatures without the need for humidification and their low cost [11,113]. Different acids, such as HNO3, H2SO4, HCl, and HClO4, can be doped into PBI membranes to act as proton donors. However, phosphoric acid (H3PO4) has been a favored dopant because compared with others, it induces higher conductivity and excellent thermal stability in PBI membranes without the need for high RH [128,129]. Jin et al. [130] developed poly(arylene pyridine) using one-step Friedel–Crafts polymerization of 4-acetylpyridine and para-terphenyl/biphenyl; the resulting membrane from the polymer exhibited excellent phosphoric acid uptake (up to 220%) with a conductivity of 0.102 S cm−1 at 180 °C. Hu et al. [131] fabricated a phosphoric acid-doped triazole-functionalized poly(arylene perfluorophenyl)-based membrane with similar conductivity at 180 °C (0.109 S cm−1), whereas Li et al. [132] synthesized poly(terphenyl pyridine) polymers using 4-acetylpyridine, p-terphenyl, and comonomer 2,2,2-trifluoroacetophenone via the acid-catalyzed polycondensation reaction. The prepared membranes using the synthesized polymer doped with phosphoric acid exhibited a proton conductivity of 0.142 S cm−1 at 160 °C under anhydrous conditions and a peak power density of 395 mW cm−2 at 180 °C in a single fuel cell fueled by non-humidified H2 and O2. The major issues with the H3PO4-doped PBI membranes are catalyst poisoning and other types of fuel cell component degradation due to acid leaching and undesirable electrochemical reactions. Another chemically different membrane is an acid–base membrane in which a non-aqueous solvent, like acid or protic ionic liquid, replaces water and acts as a proton carrier while a solid material provides the medium for proton conduction. The proton-conducting medium is mostly based on polymers with basic moieties, such as amine, amide, imide, alcohol, ether, or aprotic ionic liquid, which can react with acid to form a complex acid–base polymer membrane with proton conductivity (such as acid-doped PBI). For example, Huang et al. [133] incorporated hindered amine and imidazole in the PFSA membranes, forming an acid–base crosslinking and interpenetrating network structure for continuous proton transport and providing membranes with excellent dimensional stability and radical scavenging properties. An increase in the proton carrier acid content of the membrane can improve the proton conductivity but leads to poor mechanical stability, especially at temperatures greater than 100 °C [113].
In addition to improving the mechanical and thermal stability, the chemical modification of the membrane to incorporate radical scavenging properties has also been attempted. Wang et al. [134] grafted organic free radical scavengers, such as 2-mercapto-1-methylimidazole, 3-mercapto-1,2,4-triazole, and 2-mercaptobenzimidazole into the polyarylethersulfone backbone. Around 200 wt% phosphoric acid-doped 2-mercapto-1-methylimidazole-grafted polyarylethersulfone membranes exhibited an anhydrous proton conductivity of 78.3 mS cm−1 at 180 °C, a tensile strength of 7.8 MPa at room temperature, and a peak power density of 423 mW cm−2 at 160 °C with a fuel cell fueled by non-humidified H2 and O2. Agarwal et al. [135] incorporated phosphonic acid into Nafion membranes as a radical scavenger and compared the performance with membranes incorporated with widely the reported radical scavenger cerium. The fluoride emission rates of phosphonic acid-Nafion membranes were much lower than the baseline (non-modified Nafion) and cerium-incorporated Nafion (Ce), especially for the phosphonic acid with the shortest perfluoro chains (C6). The ratio of fluorescence intensity (I) after the addition of Fenton’s reagent with and without scavengers to the fluorescence intensity of the undegraded dye (I0) exhibited higher retention of the intensity of the fluorescent dye (I) for phosphonic acid–Nafion compared to cerium–Nafion, indicating that phosphonic acid has a higher radical scavenging capability. Even the conductivity (although not surprising given that phosphonic acids are proton conductors) and water uptake of phosphonic acid–Nafion increased. However, the strength of the phosphonic acid–Nafion decreased from baseline, while the strength of cerium–Nafion increased from baseline, although this made it more brittle, with a higher modulus and a lower elongation at break. Moreover, the Nafion membrane itself has been chemically modified to improve the radical tolerance and proton conductivity. Teixeira et al. [136] doped Nafion membranes with bisphosphonic acids and found that the doped membranes have better chemical stability after oxidative degradation under Fenton’s test conditions at 80 °C.
3. Composite PEMs
The sulfonic acid groups of PFSA membranes can undergo dynamic cross-linking with hygroscopic inorganic materials, like Al2O3, TiO2, SiO2, ZrO2, mordenite, graphene oxide, ionic liquids, and layered double hydroxide nanoparticles [113]. The dispersion of these particles in the polymer matrix enhances water retention and porosity while reducing gas crossover in the membrane [15,137]. However, incorporating hydrophilic inorganic additives into conducting membranes generally decreases proton conductivity due to the insulation effects of fillers. Numerous strategies have been employed to improve conductivity, including (i) functionalizing inorganic additives, (ii) integrating inorganic binary component materials, and (iii) introducing proton-conductive fillers [138]. Each type of inorganic particle provides specific benefits to membranes; for example, silica particles and ZrO2 reduce membrane swelling, increase water uptake, and improve proton conductivity [139,140,141]. TiO2 reduces methanol crossover, improves mechanical and thermal properties, and increases water absorption [142,143]. However, the low compatibility between polymers and fillers may impact the physical and mechanical properties of the membranes [144]. Carbon-based nanomaterials, such as carbon nanotubes [145], graphene [146], fullerenes [147], and nanodiamond [148] are promising reinforcements for improving the performance of PEM. Metal–organic frameworks (MOFs) are crystalline porous materials with three-dimensional network structures formed by organic–inorganic self-assembly [15,149]. MOFs have been extensively investigated as fillers in PEMs due to their ability to be tuned for target applications. MOFs can improve the water retention ability and proton conductivity of PFSA membranes [150]. Ionic liquids (ILs) are molten salts in the liquid state at room temperature, typically defined as liquid electrolytes composed entirely of ions without the use of solvents. Some ILs have excellent ionic conductivities even under anhydrous conditions and are, therefore, widely studied as reinforcement in membranes for PEMFC applications [151,152]. Polymeric reinforcement is another technique that has been investigated for developing composite PEMs [143]. Several polymers, such as polytetrafluoroethylene, polyvinylidene fluoride, and polybenzimidazole, have been incorporated into PSFA membranes to improve their performance [138,143].
Several nanomaterials with radical scavenging properties have been incorporated into membranes to improve radical tolerance, with cerium-based materials being the most popular [153,154,155]. Tinh et al. [156] used cerium oxide particles (5–7 nm) produced by the sol-gel method to fabricate durable layered membranes containing cerium oxide spheres on both surface layers. The smaller particles did not affect the proton conductivity of the membrane while providing radical scavenging effects. Cerium is widely used and is an effective radical scavenger; however, the migration of cerium ions to the catalyst layer during fuel cell operation causes a loss in durability and performance due to adverse effects on various components of the fuel cell. Xu et al. [157] co-doped organic antioxidant alizarin and cerium ions to fabricate PFSA composite membranes with improved oxidative stability and the reduced migration of cerium ions from the membrane. Agarwal et al. [158] used 15-Crown-5 to immobilize cerium within PFSA membranes by forming an organometallic complex of cerium with 15-Crown-5. Another way of encapsulating free radical scavengers could be fabricating a thin conductive layer on the CeOx-containing membranes [159]. Zhiyan et al. [160] changed the morphology of ceria from conventional nanoparticles to nanorods, significantly reducing the migration of cerium. Moreover, ferrocyanide, ferricyanide, and MnOx are among other radical scavenging materials being used to fabricate composite PEMs [161,162,163].
This entry is adapted from the peer-reviewed paper 10.3390/en17050998
This entry is offline, you can click here to edit this entry!
