Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
Pigmented purpuric dermatoses (PPD) encompass a group of chronic skin conditions characterized by the presence of petechiae, purpura, and pigmentation changes.
- Schamberg disease
- pigmented purpuric dermatoses
- capillaritis
1. Introduction
Pigmented purpuric dermatoses (PPD) encompass a cluster of skin disorders marked by petechial hemorrhage resulting from capillaritis. PPD are considered relatively uncommon but can affect individuals of all ages, including children [1,2]. Schamberg disease (SD) is the predominant variant observed in both adults and children [3].
1.1. Pathogenesis
The pathogenesis of PPD is not completely understood, and it may vary among the different subtypes. However, common features include inflammation and hemorrhage of superficial papillary dermal vessels, primarily capillaries. Studies suggest an immunologic event involving cell adhesion molecules (CAM) like leukocyte function adhesion 1 (LFA-1), endothelial leukocyte adhesion molecule 1 (ELAM-1), and intercellular adhesion molecule 1 (ICAM-1), indicating a cell-mediated hypersensitivity reaction [4]. The perivascular inflammatory infiltrate includes CD4+ T cells and CD1a+ dendritic cells. Cytokines produced by leukocytes may induce CAM expression, affecting fibrinolytic activity and causing intravascular fibrin deposition observed in PPD [4,5]. Capillary dilation and fragility, possibly attributed to altered function of blood vessel structure cells like fibroblasts and endothelial cells, may lead to red blood cell leakage, triggering an immune response (Figure 1). Another emerging theory suggests PPD may represent an epitheliotropic T-cell alteration, supported by observations of epidermotropism or a monoclonal pattern in the inflammatory infiltrate [6]. Progression to mycosis fungoides has been reported, emphasizing the need for integrated clinical, molecular, and histopathologic assessments for accurate diagnosis and management decisions [7,8].
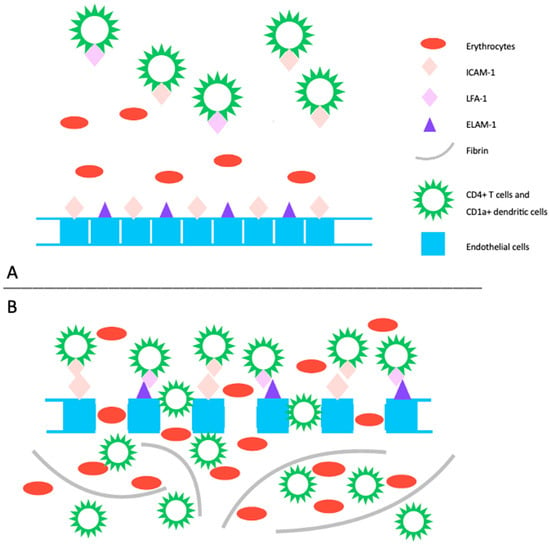
Figure 1. (A) The pathogenic mechanism of pigmented purpuric dermatoses involves a spontaneous or triggered immunologic event that induces an upregulation of cell adhesion molecules. This includes increased expression of ICAM-1 on endothelial cells, CD4+ T cells, and CD1+ dendritic cells, as well as ECAM-1 on endothelial cells, and LFA-1 on CD4+ T cells and CD1+ dendritic cells. (B) This heightened expression promotes the migration and adhesion of CD4+ T cells and CD1a+ dendritic cells, leading to increased endothelial permeability and disruption of dermal capillaries, resulting in extravasation of erythrocytes. Subsequently, this process triggers an immune response primarily mediated by T lymphocytes, leading to perivascular inflammatory infiltrate. Additionally, activated T-cells impact the plasminogen pathway, contributing to intraperivascular fibrin deposition. LFA-1: leukocyte function adhesion 1, ELAM-1: endothelial leukocyte adhesion molecule 1, ICAM-1: intercellular adhesion molecule 1.
1.2. Histopathology
Despite clinical variations, all PPD share common histopathological features, including blood vessels dilation, swelling of the endothelial cells, perivascular lymphocytic infiltration, erythrocytes extravasation, and hemosiderin-filled macrophages (Figure 2). Those features are common in all PPD, with distinctions in intensity noted in certain subtypes. Hemosiderin deposits in the superficial dermis, discerned through Perl’s and Fontana-Masson staining, differentiate PPD from stasis dermatitis [9,10]. The infiltrate predominantly consists of CD4+ lymphocytes, occasional CD1a+ dendritic cells, and macrophages [11]. Plasma cells and neutrophils may be present, with the latter observed in itching purpura lesions, such as eczematoid purpura of Doucas and Kapetanakis [11]. Mild epidermal spongiosis and lymphocytic exocytosis are common in all variants except lichen aureus, which typically shows a band-like infiltrate separated from the epidermis by a thin rim of uninvolved collagen (Grenz area) [12]. This infiltrate is also seen in pigmented purpuric lichenoid dermatosis of Gougerot and Blum [13]. A rare variant of granulomatous PPD with a superimposed hemorrhagic granulomatous infiltrate has been described as well [14]. Positive direct immunofluorescence may reveal the deposition of fibrinogen, IgM, and/or C3 in superficial dermal vessels [9]. In addition to confirming the diagnosis of pigmented purpuric eruption, a skin biopsy serves as a valuable tool for ruling out cutaneous T-cell lymphoma, especially in its early stages, as it can mimic PPD both clinically and histologically [8]. Besides a skin biopsy, it is advisable to undergo a blood test to exclude autoimmune disorders, thrombocytopenia, clotting abnormalities, and chronic infections, including HBV and HCV hepatitis [3].
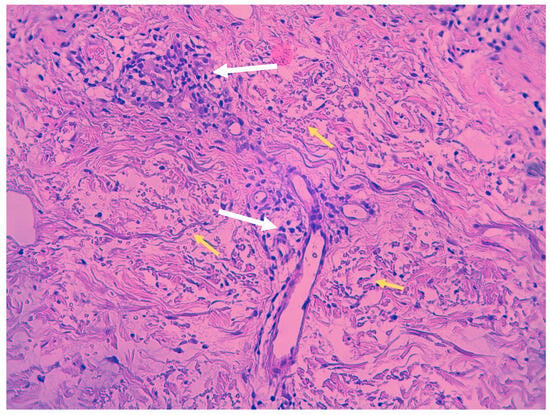
Figure 2. Histopathologic features of Schamberg disease. Lymphocytic infiltrate mainly involving capillaries (white arrows) and extravasated red blood cells (yellow arrows) (hematoxylin-eosin stain, 40× magnification).
1.3. Dermoscopy
Examining pigmented purpuric dermatoses (PPD) through dermoscopy unveils unique characteristics facilitating their diagnosis. In PPD dermoscopy, a base coloration ranging from reddish-brown to orange is evident, mirroring the underlying hemosiderin deposition. Red dots and globules, corresponding to dilated capillaries and extravasated red blood cells, are commonly observed, contributing to the characteristic purpuric appearance. Moreover, a network pattern in shades of yellowish-brown, reminiscent of branched vessels, may be discerned, underscoring the vascular nature inherent to these dermatoses [15]. Less common features include brown or red circles, grey dots, serpentine or thick linear vessels, as well as rosette structures [16] (Figure 3).
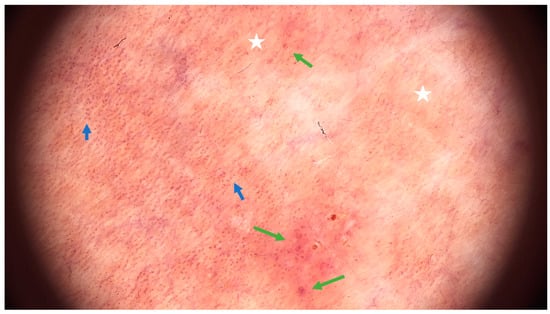
Figure 3. Dermoscopic features of Schamberg disease. Coppery-red background (white asterix), red dots (blue arrow) and globules (green arrow) (FotoFinder, FotoFinder Systems GmbH, Bad Birnbach, Germany, 20× magnification).
1.4. Differential Diagnosis
The differential diagnosis for pigmented purpuric dermatoses (PPD) includes several skin conditions that may share similar clinical or histopathological features, particularly involving the lower extremities. Prominent entities include drug hypersensitivity reactions, with potential involvement of specific medications like carbamazepine and nitroglycerine. Purpuric contact dermatitis to clothing is characterized by lesions confined to areas in contact with clothing, accompanied by intense itching, often associated with materials like wool and coloring agents. Venous stasis purpura manifests with signs of chronic venous insufficiency, including swelling, varicose veins, and venous ulcers. Other considerations encompass conditions such as purpura due to thrombocytopenia, senile purpura in the elderly, purpuric exanthema attributed to viral infections, leukocytoclastic vasculitis, IgA vasculitis (Schönlein-Henoch purpura) observed commonly in pediatric cases, Kaposi sarcoma affecting elderly or immunosuppressed individuals, and purpuric mycosis fungoides [3,10].
1.5. PPD Variants
Five classic subtypes of PPD include: progressive PPD (Schamberg disease), pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, purpura annularis telangiectodes (Majocchi disease), and eczematoid purpura of Doucas and Kapetanakis (Figure 4) [3,17]. Other less common variants include Hersch and Schwayder’s linear unilateral form of PPD (distinct from linear variants of Schamberg disease and lichen aureus) [18], quadratic form described by Higgins and Cox [19], itching purpura of Loewenthal (sometimes considered as a symptomatic variant of SD affecting adults) [20], granulomatous PPD [14], and familial PPD [21]. Furthermore, a transient, estrogen-dependent variant of PPD, called angioma serpiginosum, has been documented in the literature [22].
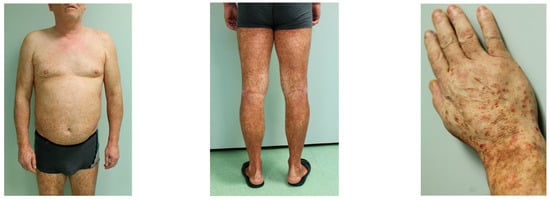
Figure 4. Clinical manifestation of Schamberg disease. Typical skin lesions include orange-red macules more prominent and coalescent on lower extremities.
2. Pigmented Purpuric Dermatoses—Treatment Options
2.1. Topical Treatment
Topical corticosteroids, commonly employed in the treatment of PPD, including Schamberg disease [2,30,36,37,38,39,40,41,42], often yield unsatisfactory effects [2,36,39,40,41,43]. Oral corticosteroids are similarly ineffective due to a high recurrence rate after dose tapering [44,45]. Although a combination of oral and topical steroids may yield better results, recurrences after treatment cessation are frequently observed [37,46]. Combinations of topical steroids with other agents, such as oral vitamin C [47] or vitamin C and rutoside [48] have been proven ineffective. Partial temporary responses were reported in patients undergoing a combined treatment of topical steroids with oral antihistaminic drugs [49] and antihistaminic drugs, vitamin C, rutoside, diosmin, and hesperidin combination [49].
Typically, topical steroids with various potency are more frequently employed in the pediatric population for PPD, serving as an alternative to oral treatment. However, prolonged use may be necessary to achieve results, necessitating consideration of potential side effects, particularly local skin atrophy and increased vascular fragility [43]. In cases of itching skin lesions, such as in purpuric lichenoid dermatitis of Gougerot–Blum, topical corticosteroids may be a viable option due to their effective and rapid improvement of pruritus [50].
Alternatively, topical calcineurin inhibitors—pimecrolimus and tacrolimus—could be considered, as they present a better side effect profile and have been proven effective in lichen aureus [51,52] and eczematoid purpura of Doucas and Kapetanakis [2], but not in pigmented purpuric lichenoid dermatosis of Gougerot and Blum [2]. Topical formulations containing vitamin K [2,42] and topical antibiotics [2,39] have proven ineffective in treating Schamberg disease and are not advisable for recommendation.
2.2. Pentoxifylline (PTX)
The effects of pentoxifylline (PTX) have been described in eight reviewed articles, and in total, the drug has been used in 187 patients [43,44,53,54,55,56,57,58]. Dosages ranging from 200 mg to 1200 mg per day were employed. Kano et al. utilized the lowest reported doses of 200–300 mg of pentoxifylline in three patients with SD. Notably, significant improvement was observed within a short period of 2 to 3 weeks, and the therapy was continued for up to 8 weeks. One patient experienced a recurrence after 4 months, which was successfully treated with another course of PTX [56]. In later studies, low doses of PTX (400 mg a day) were proven not be as effective [53,55,57,58].
Conversely, a daily dose of 1200 mg yielded a satisfactory effect in the majority of the patients, as highlighted in the reviewed articles [43,44,54]. In a comparative randomized trial, oral pentoxifylline was pitted against a topical steroid (betamethasone) [43]. Intriguingly, PTX demonstrated greater efficacy at 2 months, although this advantage diminished by the 6 month mark of treatment. Notably, there was no deterioration observed in the PTX group, while almost one in five patients in the steroid group experienced progression [43]. Taking into account this and the side effects profiles of both drugs, PTX appears to be superior to topical steroid treatment. Pentoxifylline has been also administered to a patient with acute viral hepatitis B; however, determining whether the improvement of skin lesions resulted from pentoxifylline administration or the clearance of the viral infection proves challenging [34].
Pentoxifylline is a xanthine derivative, predominantly employed for intermittent claudication associated with peripheral artery disease [59]. Presently, its applications extend to diverse conditions marked by inflammatory and vascular components. Its influence is mediated through varied mechanisms, with notable actions encompassing the inhibition and modulation of leukocytes, erythrocytes, platelets, and the vascular wall. Pentoxifylline, by exerting a non-selective inhibition of phosphodiesterase, particularly phosphodiesterase 4 (PDE-4), elevates intracellular cyclic adenosine monophosphate (cAMP) [60]. This results in the amplification of signaling pathways and downregulation of nuclear factor κB (NFκB), yielding various effects, including relaxation of vascular smooth muscle cells, enhancing blood flow; however, the precise mechanisms remain unclear [61,62].
Additionally, cAMP plays a role in suppressing the transcription of pro-inflammatory cytokines such as TNF-alpha, interleukin (IL) 1, and IL-6 [63,64]. The anti-inflammatory impact involves a reduction in macrophage and lymphocyte infiltration, inhibition of monocyte chemoattractant protein (MCP-1), decreased expression of major histocompatibility complex class II (MHC II) antigens, and the inhibition of NF-κB activation [65,66]. PTX’s hemorheological effect improves red blood cell deformability, reduces blood viscosity, and diminishes the potential for platelet aggregation and blood clot formation. [60]. In addition to the immunomodulatory effects of PPE inhibition, PTX operates through adenosine-dependent pathways, curbing the activity of immune cells during acute inflammation [67]. The antioxidant impact of PTX is proposed to stem from the preservation of glutathione levels and mitochondrial viability, as well as by neutralizing free radicals and reactive oxygen species [68,69] (Figure 5).
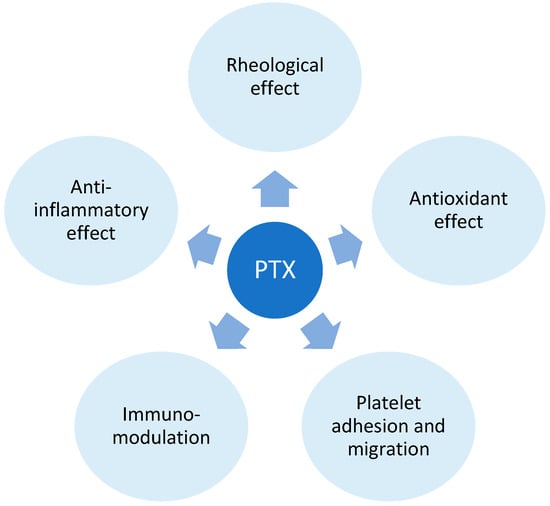
Figure 5. Pentoxifylline (PTX) mechanism of action. The mechanism of action of Pentoxifylline (PTX) involves its impact on blood viscosity, erythrocyte and platelet function, and the response of immune cells. These effects are primarily mediated by PTX’s influence on phosphodiesterase 4.
2.3. Phototherapy
Among the screened articles, psoralen and UV-A (PUVA) photochemotherapy has demonstrated successful outcomes in patients with PPD as well [37,42,70,71]. The treatment typically involved fewer than 30 sessions, with cumulative doses ranging from 29 [71] to 200 J/cm2 [42]. PUVA has also shown effectiveness in conditions such as lichen aureus [72], Majocchi disease [73], pigmented purpuric lichenoid dermatosis of Gougerot and Blum [74] and unilateral linear capillaritis [75].
Narrowband UVB therapy (UVB-NB) has proven successful in the past for treating Schamberg disease [2,37,46,49,76,77,78,79]. The majority of cases demonstrated a positive response, with only a few instances of recurrence that were effectively addressed through retreatment [78]. Maintenance therapy of approximately nine sessions over 6 weeks could be advised for achieving long-term remission [78]. In certain cases, patients may necessitate extended maintenance therapy, as exemplified by a 33-year-old male who continued UV therapy once every two weeks to proactively prevent recurrences [46]. Many reports propose a potential utility of UVB-NB in various clinical presentations of PPD [78,80,81].
Phototherapy appears to have immunomodulatory and anti-inflammatory effects that contribute to its efficacy in managing PPD [82]. Furthermore, UVB light has been identified to trigger apoptosis in epidermal and dermal lymphocytes T within psoriatic lesions [83]. UVB-NB and PUVA therapies are cost-effective, generally well tolerated, and yield favorable therapeutic outcomes. Phototherapy is a viable consideration for treating PPD, particularly in cases involving extensive skin lesions that have shown resistance to previous treatments or in pediatric patients.
2.4. Vitamin C, Flavonoids and Calcium Dobesilate
Hemorheological agents (calcium dobesilate, bioflavonoids) and vitamin C have been employed for Schamberg disease management. In the examined studies involving a total of 37 patients, the combination of 500 mg of ascorbic acid twice daily and 50 mg of rutoside twice daily led to an improvement in skin lesions or complete remission [2,45,84]. In one case where the patient received ascorbic acid, rutoside, and topical steroids, no improvement was noted, although the article did not specify the dosage and duration of the treatment [48]. Another successful combination involved vitamin C and calcium pantothenate [85]. More intricate drug combinations, such as diosmin/hesperidin/Euphorbia prostata extract/calcium dobesilate [86] or antihistaminic drug/topical steroid/ascorbic acid/rutoside/hesperidin/diosmin [49], also resulted in remission. Regarding other forms of PPD, a male patient suffering from eczematoid purpura of Doucas and Kapetanakis [87] benefited from the standard treatment combination of 500 mg of ascorbic acid twice a day and 50 mg of the bioflavonoid rutoside twice a day. Similarly, three patients with an unspecified variant of PPD also responded positively to the same treatment [88].
Vitamin C (ascorbic acid) functions as an electron donor, and serving as a reducing agent, it acts as a cofactor for numerous enzymes. In the context of treating PPD, its role lies in acting as a cofactor for hydroxylases that are integral to collagen synthesis [89]. By promoting collagen production, it enhances the strength of blood vessel walls and reduces endothelial permeability. Additionally, vitamin C diminishes local inflammation by reducing reactive oxygen species [90]. Alongside that, calcium dobesilate and bioflavonoids act as potent antioxidants, inhibiting the production of oxygen free radicals and lipid peroxidation, resulting in decreased synthesis of prostaglandins E2 or F2 and thromboxane B2 [82]. They also downregulate vascular endothelial growth factor (VEGF) expression. Flavonoids contribute to vascular health by reducing metalloproteinases (MMP) and decreasing endothelial activation, exemplified by the downregulation of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) expression. Additionally, calcium dobesilate inhibits the effects of serotonin, bradykinin, and histamine on capillary permeability. It also induces the synthesis and release of nitric oxide (NO), fostering vessel relaxation and preventing endothelial cell injury [86] (Figure 6).
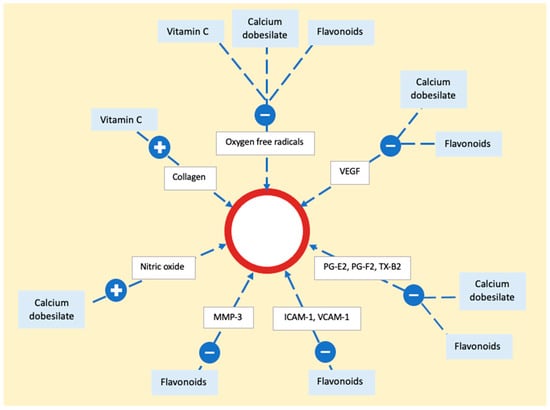
Figure 6. The mechanism of action of vitamin C, calcium dobesilate, and flavonoids on the epithelium. The process involves neutralization of oxygen free radicals by all three substances. Flavonoids and calcium dobesilate play a role in reducing the activity of vascular endothelial growth factor, prostaglandins, and thromboxane. Additionally, flavonoids contribute to a decrease in adhesion molecules and metalloproteinase, while calcium dobesilate stimulates nitric oxide synthesis. Vascular endothelial growth factor (VEGF), prostaglandin E2 (PG-E2), prostaglandin F2 (PG-F2), thromboxane B2 (TX-B2), metalloproteinase 3 (MMP-3), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1).
This entry is adapted from the peer-reviewed paper 10.3390/ijms25052644
This entry is offline, you can click here to edit this entry!
