Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Anesthesiology
Sepsis is one of the most common causes of morbidity and mortality worldwide. Despite the remarkable advances in modern medicine throughout the last century, the mortality rates associated with sepsis have remained significantly elevated, both in high- and low-income countries. The main difficulty in the diagnosis and treatment of septic patients is the tremendous heterogeneity of this condition.
- sepsis
- septic shock
- cytomics
- genomics
- epigenomics
- transcriptomics
- proteomics
- metabolomics
1. Cytomics
Cytomics refers to the study of immune cells’ responses to various sepsis-specific activators and assesses, from this perspective, the evolution of the disease.
Different studies of immune cells have revealed certain changes in sepsis patients’ neutrophils. Toxic granulation within neutrophils and the vacuolization of neutrophil cytoplasm represent the initial immune response to infectious injury in sepsis [8]. Furthermore, the structural complexity of this response is related to its magnitude. The interaction between neutrophils and T lymphocytes in sepsis is revealed in Figure 1.
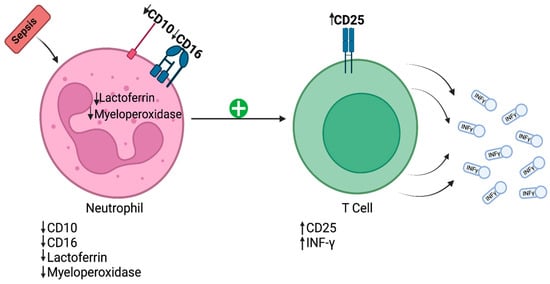
Figure 1. Initial immune response to infectious injury in sepsis. CD—cluster of differentiation; IFN-γ—interferon-gamma.
Recently, a class of granulocytes named “low-density neutrophils” (LDN) has been described [9]. According to Ran Sun et al., LDN formation is closely related to neutrophil degranulation, and these LDN provide a lower level of immune defense in sepsis compared to high-density neutrophils [9]. Aside from exhibiting a reduced phagocytic capacity and lower chemotaxis, these cells also have a longer lifespan than regular neutrophils [9]. In addition, these cells express CD15 on their surfaces and suppress T cell proliferation.
Additionally, monocytes lacking the human leucocyte antigen-DR isotype (HLA-DR) were deficient in terms of their antigen-presenting capacity and in producing TNF-α (inflammatory cytokine). T lymphocytes also have been described to overexpress inhibitory markers such as T cell immunoglobulin and mucin-domain-containing protein 1 (TIM-1), lymphocyte activation gene 3 (LAG-3), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4). T cell hyperactivation was also noticeable in afflicted patients: either through antigen-dependent activation or through an antigen-independent “bystander” activation [10].
Furthermore, the reduced expression of leukocyte-related antigen D (mHLA-DR) influences the evolution of ICU patients. Based on patients’ mHLA-DR expression variations compared to the mHLA-DR reference interval (13,000–42,000 AB/C—number of antibodies bound per monocyte), Maxime Bodinier et al. divided patients into four endotypes: “non-improvers”, “decliners”, “improvers”, and “high expressors” [11]. “Non-improver” is an endotype displaying constant expression below the mean (<4000 AB/C), while the expression in “decliners” starts at the reference values and decreases below 7500 AB/C. The “improver” endotype is characterized by an increase in mHLA-DR expression, almost reaching the reference interval, at the end of the first week. The endotype with mHLA-DR rapidly reaching the reference interval is referred to as “high expressor”. The “non-improver” and “decliner” endotypes were associated with worse outcomes: a longer length of ICU stay and exposure to invasive devices, and increased death and ICU-acquired infections, compared to the “improver” and “high expressor” endotypes [11].
Moreover, Arjun Baghela et al. showed that the progression of sepsis in certain patients was influenced by specific cellular characteristics, such as the prevalence of signaling pathways and genetic expression [12]. The neutrophilic-suppressive (NPS) endotype is associated with the activation of neutrophils, immune suppression, and the interleukin 6–signal transducer and activator of transcription (IL6/STAT) unique pathway. Studies have shown its progression to be the most severe, with the highest Sequential Organ Failure Assessment (SOFA) scores and the worst survival rate. The NPS endotype involved upregulated genes in neutrophil degranulation, NOD-like receptor (NLR) pathways, and reactive oxygen species production. It also showed the downregulation of adaptive immune and interferon signaling pathways [12].
Thus, various subcellular endotypes, reveled through cytomics study, could finally dictate the outcome in sepsis and septic shock.
2. Genomics
Genomics studies various relevant gene polymorphisms that could influence the probability of developing sepsis and septic shock and the treatment response.
Toll-like receptors (TLR) are molecules found in the membranes of various immune cells and play a crucial role in initiating the innate immune response. TLRs recognize pathogen-associated molecular patterns (PAMPs) present on bacteria, fungi, protozoa, and viruses. These PAMPs bind to TLRs and initiate the transcription of genes that code for various pro-inflammatory cytokines, chemokines, and antibody production. The main activation mechanisms rely on the NF-kB pathway. Several studies have shown that mutations in the TLR genes can lead to higher susceptibility to sepsis and septic shock [13,14,15].
NF-kB is expressed in almost all cells of the body and normally exists in the cytosol, bound to an inhibitor of the IkB family [16]. The main NF-kB-dependent mechanisms in sepsis are revealed in Figure 2.
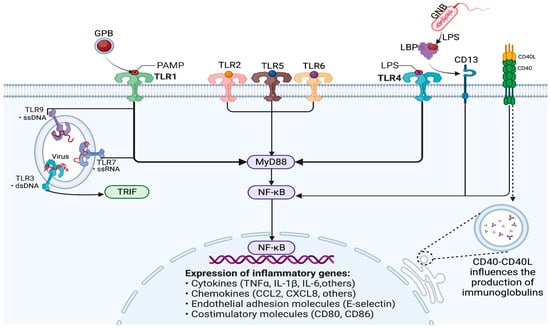
Figure 2. Modulation of immune response and gene expression through the NF-kB signaling pathway in sepsis. GPB—Gram-positive bacteria; GNB—Gram-negative bacteria; PAMP—pathogen-associated molecular pattern; TLR—Toll-like receptor; LPS—lipopolysaccharide; LBP—lipopolysaccharide-binding protein; NF-kB—nuclear factor kappa B; MyD88—myeloid differentiation primary response 88; TRIF-TIR—Toll/interleukin-1 receptor domain-containing adaptor protein inducing interferon beta, also known as TICAM-1 (TIR-containing adaptor molecule 1); ssDNA—single-stranded DNA; dsDNA—double-stranded DNA; ssRNA—single-stranded RNA; CD—cluster of differentiation; CD40L—CD40 ligand; TNF-α—tumor necrosis factor alpha; IL—interleukin; CCL2—chemokine (C-C motif) ligand 2; CXCL2—C-X-C motif chemokine ligand 8.
CD40 is a receptor protein expressed by various immune cells. The CD40 ligand (CD40L) binds to the CD40 receptor on B cells and dendritic cells and thus influences the production of immunoglobulins and, consequently, the probability of developing sepsis [17].
Variability in the genes responsible for the NF-kB pathway can influence mortality and morbidity in septic shock. In a retrospective study, genotyping single-nucleotide polymorphisms in a couple of genes allowed the definition of the CC genotype of NIK rs7222094, which was demonstrated to be associated with increased mortality and more renal and hematological failure than the CT and TT genotypes (T—mutated allele, C—normal allele). Moreover, an important chemokine, CXCL10, released by activating NF-kB, had lower levels in the CC genotype in septic shock patients. Thus, polymorphisms in the NF-kB genes could predict the risk of death or the response to target therapies [18].
A recent study led by Sun J. et al. aimed to illustrate the correlation between single-nucleotide polymorphisms (SNPs) of the NFKB1 gene and sepsis-associated acute kidney injury (AKI) [19]. The result showed that the carrier group of SNPs (A-allele carriers) on the rs41275743 and rs4648143 loci of the NFKB1 gene was associated with a risk of developing AKI 1.46 that was 1.56 times higher than the risk of the cluster lacking SNPs (G-allele carriers). Moreover, Thair et al. studied the influence of NF-kB genetic variation on the outcome of septic shock [20]. The results revealed that a single-nucleotide polymorphism (SNP) in the NF-kB-inducing kinase was significantly associated with increased chances of renal and hematological dysfunction and with greater 28-day mortality. The degree of NF-kB activation is also linked to illness severity [21]. On the other hand, it was also shown that a deficit in the activation of NF-kB in sepsis is a factor involved in inducing complications (acute lung injury) and increasing the illness severity [22].
In septic shock, there are several mechanisms that affect the vasomotor tonus of blood vessels, leading to systemic hypotension. The main mechanisms and their pharmacological modulation are revealed in Figure 3.
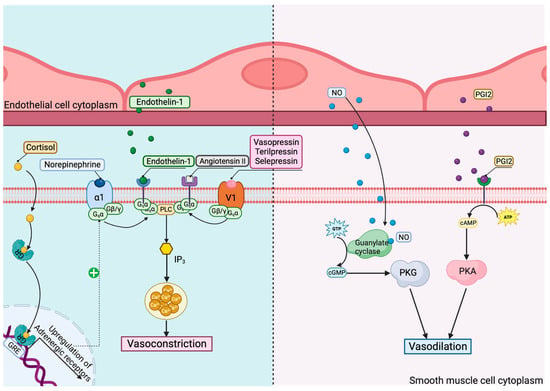
Figure 3. Mechanisms and pharmacological modulation of vasomotor dysregulation in septic shock. GR—glucocorticoid receptor; GRE—glucocorticoid response elements; V1—vasopressin receptor 1; α1—α1 adrenoreceptor; PLC—phospholipase C; IP3—inositol trisphosphate; NO—nitric oxide; PGI2—prostaglandin I2; PKG—protein kinase G; PKA—protein kinase A.
Consequently, a differentiated genomic-targeted approach for vasopressor therapy in septic shock should be applied. Norepinephrine is the first choice in septic shock [1]. In some cases, such as septic patients with catecholamine-refractory shock, the treatment of choice should be reconsidered, with some alternatives being the catecholamine-sparing agent arginine vasopressin (AVP), corticosteroids, and angiotensin II. Terlipressin (TP), the long-lasting analog of vasopressin, has higher affinity for V1 receptors, which mediate vasoconstriction; thus, it might be considered a more optimized therapeutic option. Selepressin is also an agonist for vasopressin and an effective substitute for norepinephrine. Angiotensin II, a critical regulator of the blood volume and systemic vascular resistance, has proven efficient when administered together with norepinephrine, because it decreases the dosage of catecholamine necessary while avoiding its unwanted effects [23,24].
An important risk factor in norepinephrine- as well as vasopressin-treated patients is the presence of single-nucleotide polymorphisms (SNPs) that affect their receptors. Thus, a genomic-based approach involving key receptor polymorphisms might influence the therapeutic strategy and outcome in patients with refractory septic shock. Nakada et al. studied the influence of ADRB2 (gene for β2-adrenergic receptor) polymorphisms on the outcomes of septic shock treated with norepinephrine [25]. Their study revealed that a certain ADRB2 gene polymorphism was associated with greater cardiovascular dysfunction, organ dysfunction, and mortality in patients with septic shock. They described three frequent single-nucleotide polymorphisms: Cys/Arg-19, associated with altered gene translation in vitro, and Gly/Arg16 and Gln/Glu27, responsible for receptor expression in vitro and receptor desensitization in vivo. Homozygotes for this Cys/Gly/Gln haplotype are known as the AA genotype of the ADRB2 rs104271 and this was correlated with increased 28-day mortality, more organ failure, a higher heart rate in the first 5 days, and higher norepinephrine administration in septic patients (decreased receptor expression, decreased anti-inflammatory effect) [25].
According to the VASST study, the autosomal dominant form of rs28418396 SNP is a risk factor for serious adverse events (SAEs; associated with the use of vasoconstrictor therapy) [26]. The identified SNP is located near the 5’-UTR of the gene responsible for coding arginine vasopressin receptor 1B (AVPR1b), whose stimulation releases stress hormones, like cortisone. Additionally, a higher SAE probability was identified in patients with the AA genotype of rs28418396 compared to those with the TT/TA genotype.
Aside from this mechanism, glucocorticoids impact the vascular tone and have a pivotal role in septic shock [27]. According to De Kloet et al, SNPs in the glucocorticoid receptor (GR) impact the metabolic profile and cardiovascular parameters: ER22/23EK, with a favorable profile (decreased the response to cortisol), and N363S together with Bcl1, with a less advantageous one (they increased the cortisol responses to various types of stress, causing immunosuppression in the patient and a predisposition to infection) [28]. Once sepsis is induced, the immunosuppressive effect of glucocorticoids is beneficial, reducing the transcription of pro-inflammatory genes via the inhibition of nuclear factor kappa B [27]. The hGR NS-1 form, described by Baker et al., contains three nonsynonymous SNPs (A214G, T962C, and A2297G) [29]. The presence of either SNP A214G or T962C resulted in a decreased response to dexamethasone in sepsis. However, if methylprednisolone was administered, the presence of SNP A214G resulted in greater activity when compared with hGR, whereas T962C resulted in activity equivalent to hGR [29].
Thus, the interindividual variability of the mechanisms that modulate the NF-kB-mediated immune response and catecholamine-responsive vascular tone, revealed by genomic studies, could contribute to personalized medicine approaches in sepsis and septic shock.
This entry is adapted from the peer-reviewed paper 10.3390/jpm14030225
This entry is offline, you can click here to edit this entry!
