Bidirectional communication between the gut and brain is primarily mediated by the nervous, immune, and endocrine pathways [
10,
11]. Nervous pathways include the enteric nervous system (ENS), autonomic nervous system (ANS), and vagus nerve. Visceral afferents normally transmit sensory information from the gut to the CNS, which, in turn, modulates GI function by efferent signals. Intestinal motility, absorption, secretion, and blood flow are all regulated by the ANS [
11]. Interestingly, the ANS shares several mediators and receptors with the gut’s immune system and, thus, can also play a role in regulating local inflammatory processes [
12]. Signaling in the immune pathways is primarily mediated by enterocytes, resident immune cells, and the gut microbiota. Enterocytes, particularly a specialized subset called tuft cells, and gut-associated immune cells, such as macrophages, neutrophils, and dendritic cells, all express toll-like receptors (TLRs) that can recognize molecular patterns on the surfaces of invading pathogens and initiate an innate immune response [
11]. Upon activation, these cells release inflammatory cytokines and chemokines that not only aid in further immune cell recruitment but also trigger signaling cascades that can communicate with the CNS. Cytokines may also act locally on vagal afferents [
10] and subsequently affect signaling within the GBA. Endocrine pathways mainly involve hormones and the hypothalamic–pituitary–adrenal (HPA) axis. However, neuroactive microbial products, such as short-chain fatty acids (SCFAs) and secondary bile acids (2-BAs), can also propagate signals to the CNS either directly, by accessing the body’s systemic circulation, or indirectly, by interacting with enteroendocrine, enterochromaffin, and immune cells of the gut [
10].
3. Evidence of Gut Dysbiosis in Human Amyotrophic Lateral Sclerosis
The human gut is habitat to a highly complex yet balanced network of commensal microorganisms spanning all three domains of life: bacteria, archaea, and eukarya (including fungi, yeasts, and protozoa) [
13]. Bacteriophages and eukaryotic viruses are also integral components of the gut microbiome [
14]. Although the exact composition and diversity of microbial communities throughout the GI tract vary considerably, the gut microbiome is primarily dominated by bacterial populations. Specifically, among the eight identified bacterial phyla in the human gut, Firmicutes and Bacteroidetes constitute the majority portion and represent over 90% of all intestinal microbiota [
13,
15]. The remaining 10% is typically composed of Actinobacteria and smaller proportions of Proteobacteria, Verrucomicrobia, or Cyanobacteria [
13,
15,
16]. The division of the intestinal flora at this taxonomic level is similar, if not uniform, across most healthy humans. However, each individual possesses a unique microbiome made up of different species and strains at varying densities likely owing to a number of genetic factors and host–microbe interactions [
17,
18]. Age, diet, lifestyle, environment, and disease may also account for individualized differences since they can shape and alter the gut’s microbial composition [
15].
Alterations to the gut flora are typically examined via high-throughput sequencing techniques such as 16S rRNA sequencing and shotgun metagenomics. Bioinformatic analyses may also be used to assess the taxonomic composition or diversity of detected microbial communities in various samples, as well as across different patient groups. To date, the largest human gut profiling study was conducted by Guo et al. [
26] and included a total of 185 participants. The fecal microbiome of ALS patients and unrelated healthy controls were longitudinally compared at three different time points. Two bacterial phyla, Firmicutes and Cyanobacteria, were significantly different in relative abundance between patients and controls at the first collection point (baseline) [
26]. Adjustment for confounding factors (sex, age, and body mass index) further highlighted alterations to the abundance of six specific genera:
Bacteroides,
Parasutterella, and
Lactococcus were all significantly enriched in ALS samples, while
Faecalibacterium and
Bifidobacterium were markedly reduced compared to controls. The presence of a distinct gut microbiome in ALS patients was further validated by significant differences in the beta-diversity between the two study groups. Interestingly, only the abundance of Firmicutes significantly varied at the second time point [
26], suggesting that the gut microbiome may continue to undergo changes over the course of the disease.
Other human microbiome studies also provide evidence of dysbiosis in ALS patients. For instance, Fang et al. [
28] demonstrated several significant alterations to the gut microbiome at different taxonomic levels. Bacteroidetes (phylum), Bacteroidia (class), Bacteroidales (order), and
Dorea (genus) were all enriched in ALS samples compared to healthy controls, whereas Firmicutes (phylum), Clostridia (class),
Lachnospiraceae (family),
Oscillibacter (genus), and
Anaerostipes (genus) were notably decreased. Di Gioia et al. [
29] reported that
Escherichia coli,
Clostridiales Family XI (family),
Gastranaerophilalaes (family), and Cyanobacteria (phylum) were all significantly elevated in ALS, while
Clostridiaceae 1 (family) was lower in patients than controls. While the total bacterial count did not differ between the two study groups, ALS stool samples showed lower DNA concentrations compared to controls, possibly due to significantly decreased amounts of yeast [
29]. Patients with reduced yeast counts were significantly correlated with lower ALSFRS-R scores and forced vital capacity percentage (FVC%). Thus, shifts in various microbial populations, not only bacteria, may impact disease progression or clinical manifestation.
Given that caregivers who live or closely interact with ALS patients may share environmental exposures, and changes to the gut microbiome by extension, several studies use healthy family members and spouses as controls. Niccolai et al. [
31], for example, compared the stool samples of ALS patients with those collected from cohabiting controls and found they were distinct. Patient samples showed a significant enhancement in the relative abundance of
Senegalimassilia (genus), while
Subdoligranulum (genus) and several members of
Lachnospiraceae (family) were instead only elevated in family members. Among others,
Adlercreutzia,
Lachnospiraceae_FCS020_group, and
Romboutsia were further correlated with disease progression and survival in the ALS group [
31]. Patients with a slow progression rate, in particular, were associated with a higher abundance of
Streptococcaceae (family) but a significant reduction in fecal α-diversity [
31].
While most human microbiome studies present evidence of gut dysbiosis in ALS patients, some conflicting findings have been reported. Inconsistencies with regards to differences in the alpha/beta-diversity or Firmicutes/Bacteroidetes (F/B) ratio are amongst the most common. The F/B ratio is widely accepted as a sign of intestinal homeostasis and overall gut health, and its alteration has been previously described in inflammatory bowel diseases [
33,
34] as well as metabolic disorders [
35,
36,
37]. However, the precise implications or effects of F/B imbalance on disease progression and patient outcome in ALS remain unknown due to conflicting results amongst current profiling studies.
4. Gut Dysbiosis Contributes to Pathology in Amyotrophic Lateral Sclerosis
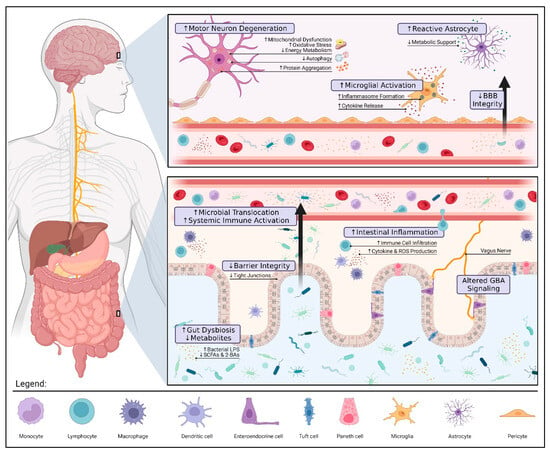
Changes to the gut microbiota can influence distant organs by direct (e.g., translocation) and indirect (e.g., immune dysregulation) means. While the precise mechanisms are still under investigation, several connections between gut dysbiosis and ALS pathology have been identified (see Figure 1). It is important to highlight that further research is needed to definitively determine whether these findings are causal.
Figure 1. Gut Dysbiosis and Pathology in Amyotrophic Lateral Sclerosis. Alterations to the gut microbiota can contribute to ALS pathology via three pivotal mechanisms: compromised gut barrier integrity, metabolic dysfunction, and immune dysregulation. Reduced expression of tight junctions along the intestinal epithelium allows for microbial invasion and subsequent inflammation. These microbes may also translocate into the blood, triggering a systemic immune response (e.g., endotoxemia, proinflammatory cytokine production, and peripheral monocyte activation). If prolonged, systemic inflammation can damage the blood–brain barrier (BBB) and result in the overactivation of microglia and astrocytes further aggravating neuroinflammation. The loss of immunomodulatory and neuroprotective metabolites such as butyrate, a short-chain fatty acid (SCFA), promotes motor neuron degeneration by increased oxidative stress and mitochondrial dysfunction. The interplay between gut and brain health highlights the therapeutic potential of gut–brain axis (GBA) modulation in ALS. Restoring a healthy microbial balance may not only alleviate patient symptoms by modulating these mechanisms but also prolong survival by mitigating disease progression. The figure was created using
Biorender.com.
4.1. Dysbiosis and Intestinal Barrier Integrity
The intestinal mucosa and its components serve as the gut’s frontline defense system against invasion by harmful pathogens and toxins. Healthy microbiomes are essential to the function and integrity of this barrier [
49]. A common way by which commensal microorganisms fortify the gut’s lining mucosa is by direct upregulation of intercellular junctions. Alvarez et al. [
50] demonstrate that several commensal strains of
Escherichia coli can promote the translation and redistribution of tight junction proteins, such as zonula occludens (ZO)-1 and claudin-2.
Alterations in the microbial composition of the gut can disrupt intercellular junctions, and by extension, increase mucosal permeability (a phenomenon commonly referred to as “leaky gut”). A recent study by Wu et al. [
52] shows that gut dysbiosis was associated with compromised barrier integrity in a transgenic mouse model of ALS. Compared to wild-type mice, the relative abundance of
Fermicus,
Escherichia coli, and
Butyrivibrio Fibrisolvens, a butyrate-producing bacteria, were all markedly lower in transgenic mice. These shifts occurred before ALS symptom onset (at 2 months of age) and were associated with a significant reduction in colonic expression of tight (ZO-1) and adherent (E-cadherin) junction proteins [
52].
4.2. Dysbiosis and Metabolic Dysfunction
Motor neurons are remarkably vulnerable to systemic and cellular disturbances in energy homeostasis. Impaired mitochondrial function, oxidative stress, and altered glucose metabolism have therefore all been implicated in the pathogenesis of ALS [
60]. Given that the gut microbiota significantly regulates nutrient availability and bioenergetics [
61], recent evidence suggests that dysbiosis may drive metabolic dysfunction in ALS. Sagi et al. [
62], for example, report that, in mice lacking the antioxidant enzyme superoxide dismutase 1 (SOD1), a well-established animal model of ALS, changes to the gut microbiota and F/B ratio were associated with significant metabolic dysfunction. Increased oxidative stress caused by SOD1 deficiency not only suppressed hepatic gluconeogenesis but also promoted lipid accumulation (causing fatty liver in young 15-week-old mice). Moreover, redox imbalance was associated with the increased nitrosylation and subsequent inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a crucial enzyme in glycolysis [
62]. If not compensated, chronic shifts in carbohydrate metabolism can have detrimental impacts on energy homeostasis and disease progression in ALS [
63].
4.3. Dysbiosis and Immune Dysregulation
Dysregulation of both central and peripheral immune systems has been previously described in ALS [
70]. Persistent inflammation in the brains and spinal cords of ALS patients is typically associated with an increase in the number of reactive microglia and astrocytes. While initially neuroprotective [
71], chronic glial cell activation exacerbates inflammation by promoting inflammasome formation and the production of several proinflammatory cytokines, such as IL-1β and IL-18 [
70,
72]. Impaired autophagy and the loss of metabolic support normally provided by astrocytes can also contribute to neuronal cell injury and degeneration in ALS [
72]. Although many of the previously discussed profiling studies link alterations in the gut microbiota to neuroinflammation in ALS, the precise mechanisms are not fully understood. Some studies suggest immune dysregulation following dysbiosis is an indirect consequence of endotoxemia and an altered SCFA metabolism. Among its many anti-inflammatory activities, butyrate can inhibit histone deacetylase and subsequently shift microglial polarization towards an anti-inflammatory and more neuroprotective phenotype [
73,
74]. It can also suppress IL-17 to maintain the balance between regulatory T cells (Treg) and T helper 17 cells (Th17) for systemic immune response control [
75]. In ALS patients, the levels of butyrate-producing bacteria are reported to be significantly lower compared to healthy controls [
47]. These alterations not only impact SCFA production but can also exacerbate local gut inflammation and trigger a systemic or neuroinflammatory response.
5. Gut-Microbiota-Modulating Agents in Amyotrophic Lateral Sclerosis
5.1. Prebiotics
Prebiotics include a wide range of dietary fibers and compounds that can effectively stimulate the growth or activity of beneficial gut microbes [
82,
83]. Although initially indigestible, prebiotics undergo fermentation in the colon to produce metabolites (e.g., SCFAs) that help regulate systemic metabolism and promote overall gut health [
84]. In a recent study by Zhang et al. [
85], oral administration of the prebiotic galacto-oligosaccharides (GOS) not only increased the relative abundance of
Lactobacillus but also attenuated neuroinflammation and cognitive impairment in transgenic AD mice. The use of fructo-oligosaccharides (FOS), alone or in combination with GOS, similarly promoted
Bifidobacterium growth and alleviated AD pathology. The effects of these prebiotics were partly attributed to the downregulation of signaling pathways shared between the colons and cortices of mice [
85], suggesting that GBA modulation could significantly influence CNS pathology. Preclinical evidence on the use of prebiotics in ALS remains preliminary and inconclusive.
5.2. Probiotics
Probiotics comprise a number of live microorganisms, often beneficial gut bacteria and yeast, that play a crucial role in maintaining the body’s microbial balance and promoting well-being [
88]. Many of the currently well-recognized probiotics belong to the
Lactobacillus,
Bifidobacterium,
Saccharomyces,
Enterococcus, and
Streptococcus genera [
88]. Several studies have explored the potential of specific strains in treating neurodegenerative disorders. For example, Zhu et al. [
89] showed that the administration of
Bifidobacterium breve significantly attenuated neuroinflammation, amyloid deposition, and cognitive impairment in APP/PS1 transgenic mice. These changes were associated with increased regulation of gut microbiota composition and improvement in intestinal barrier function [
89], highlighting the therapeutic potential of GBA modulation. Another study similarly reported
Bifidobacterium breve supplementation in amyloid beta precursor protein (APP) knock-in mice increased the bioavailability of anti-oxidative metabolites, which improved cognitive function [
90].
Aligned with the aforementioned findings, probiotic interventions in ALS have been shown to be neuroprotective. A recent study by Labarre et al. [
94] investigated the effects of sixteen different probiotic formulations on
Caenorhabditis elegans strains that were genetically modified to express two human ALS-associated proteins: fused in sarcoma (FUS) and TAR DNA-binding protein 43 (TDP-43). Although most combinations had little to no effect, treatment with the probiotic
Lacticaseibacillus rhamnosus HA-114 alone was effective in delaying neurodegeneration and preventing paralysis [
94]. These effects were observed in FUS and TDP-43 mutant worms but not wild-type strains, suggesting that the benefits of HA-114 were more specifically tied to ALS pathology.
5.3. Postbiotics
Postbiotics are non-viable, biologically active components or metabolic byproducts of the gut microbiota [
84]. Common examples of postbiotics are functional proteins, extracellular polysaccharides, bacterial lysates, and fermentation byproducts (e.g., SCFAs). In ALS, Zhang et al. [
69] have shown that the natural bacterial product, butyrate, was neuroprotective when given at a 2% concentration in filtered drinking water to SOD1-G93A transgenic mice. Treatment with postbiotics not only restored intestinal microbial homeostasis and gut barrier integrity, but also delayed ALS progression and prolonged the lifespan of mice. Moreover, abnormal Paneth cell accumulation and SOD1 mutant protein aggregation were significantly lowered in the intestines of mice receiving butyrate compared to the control groups [
69]. These findings suggest that the therapeutic potential of postbiotics particularly lies in their ability to address gut-related abnormalities and inflammation.