The growth of antibiotic-resistant bacterial infections necessitates focusing on host-derived immunotherapies. γδ T cells are an unconventional T cell subset, making up a relatively small portion of healthy circulating lymphocytes but a substantially increased proportion in mucosal and epithelial tissues. γδ T cells are activated and expanded in response to bacterial infection, having the capability to produce proinflammatory cytokines to recruit neutrophils and clear infection. They also play a significant role in dampening immune response to control inflammation and protecting the host against secondary challenge, making them promising targets when developing immunotherapy. Importantly, γδ T cells have differential metabolic states influencing their cytokine profile and subsequent inflammatory capacity.
- γδ T cells
- immunometabolism
- bacterial infection
- Staphylococcus aureus
1. Introduction
2. Immunometabolism of γδ T Cells during Bacterial Infection
3. The Site-Specific γδ T Cell Response to Staphylococcus aureus Infection
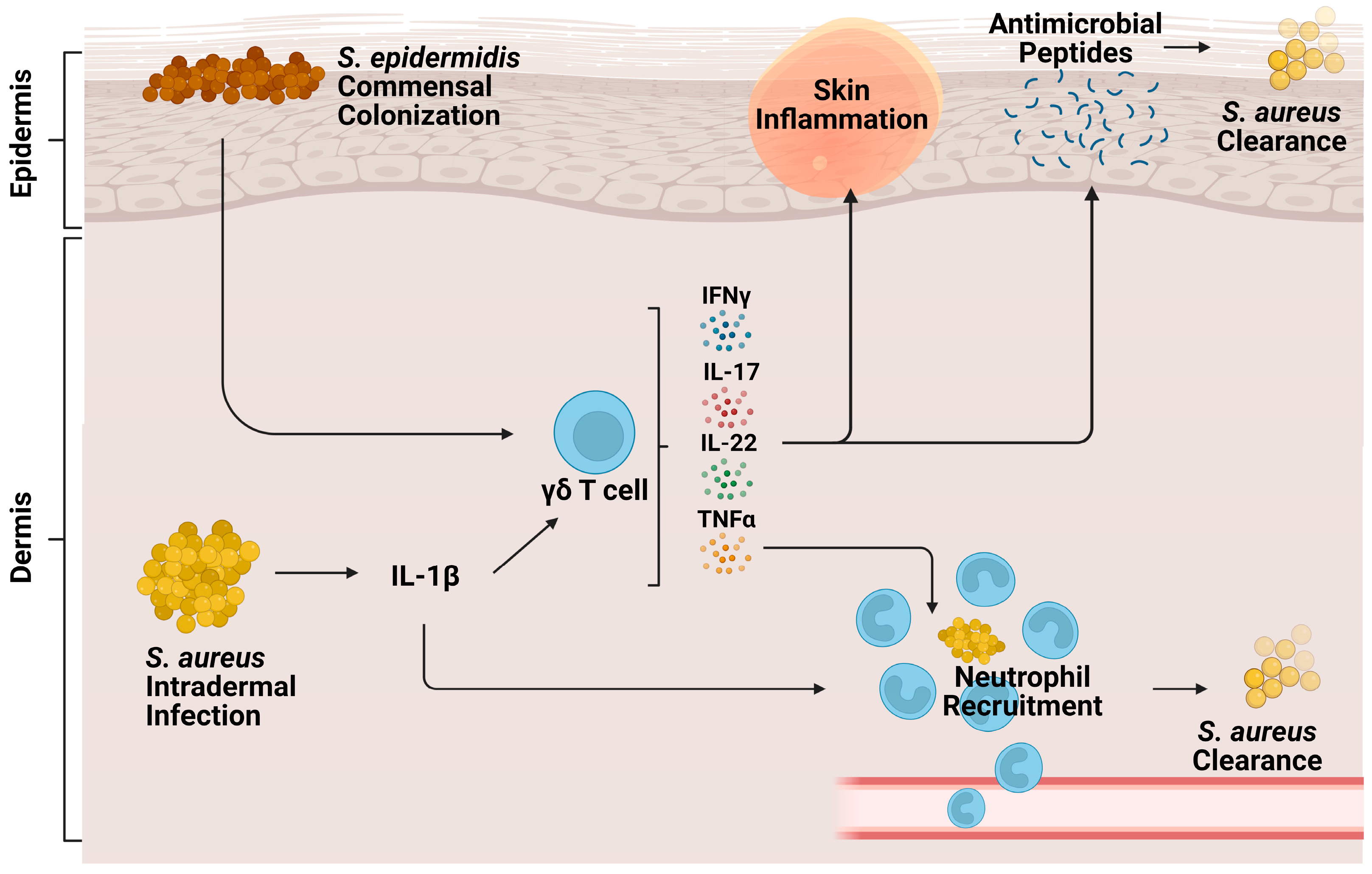
3.1. Cutaneous Infection
3.2. Pneumonia
3.3. Peritonitis
This entry is adapted from the peer-reviewed paper 10.3390/biom14020225
References
- Carding, S.R.; Egan, P.J. γδ T Cells: Functional Plasticity and Heterogeneity. Nat. Rev. Immunol. 2002, 2, 336–345.
- Kabelitz, D.; Wesch, D.; Hinz, T. Gamma Delta T Cells, Their T Cell Receptor Usage and Role in Human Diseases. In Springer Seminars in Immunopathology; Springer International: Berlin, Germany, 1999; Volume 21, pp. 55–75.
- Brown, A.F.; Murphy, A.G.; Lalor, S.J.; Leech, J.M.; O’Keeffe, K.M.; Mac Aogáin, M.; O’Halloran, D.P.; Lacey, K.A.; Tavakol, M.; Hearnden, C.H.; et al. Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection. PLoS Pathog. 2015, 11, e1005226.
- Mayassi, T.; Barreiro, L.B.; Rossjohn, J.; Jabri, B. A Multilayered Immune System through the Lens of Unconventional T Cells. Nature 2021, 595, 501–510.
- Wesch, D. Analysis of the TCR Vgamma Repertoire in Healthy Donors and HIV-1-Infected Individuals. Int. Immunol. 1998, 10, 1067–1075.
- Lee, D.; Rosenthal, C.J.; Penn, N.E.; Dunn, Z.S.; Zhou, Y.; Yang, L. Human γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy. Cancers 2022, 14, 3005.
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. J. Immunol. Res. 2018, 2018, 5081634.
- Davey, M.S.; Lin, C.-Y.; Roberts, G.W.; Heuston, S.; Brown, A.C.; Chess, J.A.; Toleman, M.A.; Gahan, C.G.M.; Hill, C.; Parish, T.; et al. Human Neutrophil Clearance of Bacterial Pathogens Triggers Antimicrobial γδ T Cell Responses in Early Infection. PLoS Pathog. 2011, 7, e1002040.
- Eberl, M.; Roberts, G.W.; Meuter, S.; Williams, J.D.; Topley, N.; Moser, B. A Rapid Crosstalk of Human γδ T Cells and Monocytes Drives the Acute Inflammation in Bacterial Infections. PLoS Pathog. 2009, 5, e1000308.
- Leslie, D.S.; Vincent, M.S.; Spada, F.M.; Das, H.; Sugita, M.; Morita, C.T.; Brenner, M.B. CD1-Mediated γ/δ T Cell Maturation of Dendritic Cells. J. Exp. Med. 2002, 196, 1575–1584.
- Skeen, M.J.; Freeman, M.M.; Ziegler, H.K. Changes in Peritoneal Myeloid Populations and Their Proinflammatory Cytokine Expression during Infection with Listeria monocytogenes Are Altered in the Absence of Gamma/Delta T Cells. J. Leukoc. Biol. 2004, 76, 104–115.
- Cai, Y.; Xue, F.; Fleming, C.; Yang, J.; Ding, C.; Ma, Y.; Liu, M.; Zhang, H.; Zheng, J.; Xiong, N.; et al. Differential Developmental Requirement and Peripheral Regulation for Dermal Vγ4 and Vγ6T17 Cells in Health and Inflammation. Nat. Commun. 2014, 5, 3986.
- Vermijlen, D.; Prinz, I. Ontogeny of Innate T Lymphocytes—Some Innate Lymphocytes Are More Innate than Others. Front. Immunol. 2014, 5, 486.
- Sutoh, Y.; Mohamed, R.H.; Kasahara, M. Origin and Evolution of Dendritic Epidermal T Cells. Front. Immunol. 2018, 9, 1059.
- Bertram, T.; Reimers, D.; Lory, N.C.; Schmidt, C.; Schmid, J.C.; Heinig, L.; Bradtke, P.; Rattay, G.; Zielinski, S.; Hellmig, M.; et al. Kidney-Resident Innate-like Memory γδ T Cells Control Chronic Staphylococcus aureus Infection of Mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2210490120.
- Fiala, G.J.; Gomes, A.Q.; Silva-Santos, B. From Thymus to Periphery: Molecular Basis of Effector γδ-T Cell Differentiation. Immunol. Rev. 2020, 298, 47–60.
- Zheng, J.; Liu, Y.; Lau, Y.-L.; Tu, W. γδ-T Cells: An Unpolished Sword in Human Anti-Infection Immunity. Cell. Mol. Immunol. 2013, 10, 50–57.
- Murphy, A.G.; O’Keeffe, K.M.; Lalor, S.J.; Maher, B.M.; Mills, K.H.G.; McLoughlin, R.M. Staphylococcus aureus Infection of Mice Expands a Population of Memory γδ T Cells That Are Protective against Subsequent Infection. J. Immunol. 2014, 192, 3697–3708.
- Sheridan, B.S.; Romagnoli, P.A.; Pham, Q.-M.; Fu, H.-H.; Alonzo, F.; Schubert, W.-D.; Freitag, N.E.; Lefrançois, L. γδ T Cells Exhibit Multifunctional and Protective Memory in Intestinal Tissues. Immunity 2013, 39, 184–195.
- Romagnoli, P.A.; Sheridan, B.S.; Pham, Q.-M.; Lefrançois, L.; Khanna, K.M. IL-17A-Producing Resident Memory γδ T Cells Orchestrate the Innate Immune Response to Secondary Oral Listeria monocytogenes Infection. Proc. Natl. Acad. Sci. USA 2016, 113, 8502–8507.
- Morita, C.T.; Jin, C.; Sarikonda, G.; Wang, H. Nonpeptide Antigens, Presentation Mechanisms, and Immunological Memory of Human Vγ2Vδ2 T Cells: Discriminating Friend from Foe through the Recognition of Prenyl Pyrophosphate Antigens. Immunol. Rev. 2007, 215, 59–76.
- Liuzzi, A.R.; Kift-Morgan, A.; Lopez-Anton, M.; Friberg, I.M.; Zhang, J.; Brook, A.C.; Roberts, G.W.; Donovan, K.L.; Colmont, C.S.; Toleman, M.A.; et al. Unconventional Human T Cells Accumulate at the Site of Infection in Response to Microbial Ligands and Induce Local Tissue Remodeling. J. Immunol. 2016, 197, 2195–2207.
- Morita, C.T.; Li, H.; Lamphear, J.G.; Rich, R.R.; Fraser, J.D.; Mariuzza, R.A.; Lee, H.K. Superantigen Recognition by γδ T Cells. Immunity 2001, 14, 331–344.
- Vermijlen, D.; Gatti, D.; Kouzeli, A.; Rus, T.; Eberl, M. γδ T Cell Responses: How Many Ligands Will It Take till We Know? In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 84, pp. 75–86.
- Rosenberg, G.; Riquelme, S.; Prince, A.; Avraham, R. Immunometabolic Crosstalk during Bacterial Infection. Nat. Microbiol. 2022, 7, 497–507.
- Jensen, K.D.C.; Su, X.; Shin, S.; Li, L.; Youssef, S.; Yamasaki, S.; Steinman, L.; Saito, T.; Locksley, R.M.; Davis, M.M.; et al. Thymic Selection Determines γδ T Cell Effector Fate: Antigen-Naive Cells Make Interleukin-17 and Antigen-Experienced Cells Make Interferon γ. Immunity 2008, 29, 90–100.
- Ribot, J.C.; deBarros, A.; Pang, D.J.; Neves, J.F.; Peperzak, V.; Roberts, S.J.; Girardi, M.; Borst, J.; Hayday, A.C.; Pennington, D.J.; et al. CD27 Is a Thymic Determinant of the Balance between Interferon-γ- and Interleukin 17–Producing γδ T Cell Subsets. Nat. Immunol. 2009, 10, 427–436.
- Zuberbuehler, M.K.; Parker, M.E.; Wheaton, J.D.; Espinosa, J.R.; Salzler, H.R.; Park, E.; Ciofani, M. The Transcription Factor C-Maf Is Essential for the Commitment of IL-17-Producing γδ T Cells. Nat. Immunol. 2019, 20, 73–85.
- Lopes, N.; McIntyre, C.; Martin, S.; Raverdeau, M.; Sumaria, N.; Kohlgruber, A.C.; Fiala, G.J.; Agudelo, L.Z.; Dyck, L.; Kane, H.; et al. Distinct Metabolic Programs Established in the Thymus Control Effector Functions of γδ T Cell Subsets in Tumor Microenvironments. Nat. Immunol. 2021, 22, 179–192.
- Chen, X.; Cai, Y.; Hu, X.; Ding, C.; He, L.; Zhang, X.; Chen, F.; Yan, J. Differential Metabolic Requirement Governed by Transcription Factor C-Maf Dictates Innate γδT17 Effector Functionality in Mice and Humans. Sci. Adv. 2022, 8, eabm9120.
- Frascoli, M.; Ferraj, E.; Miu, B.; Malin, J.; Spidale, N.A.; Cowan, J.; Shissler, S.C.; Brink, R.; Xu, Y.; Cyster, J.G.; et al. Skin gδ T Cell Inflammatory Responses Are Hardwired in the Thymus by Oxysterol Sensing via GPR183 and Calibrated by Dietary Cholesterol. Immunity 2023, 56, 562–575.e6.
- Xia, X.; Cao, G.; Sun, G.; Zhu, L.; Tian, Y.; Song, Y.; Guo, C.; Wang, X.; Zhong, J.; Zhou, W.; et al. GLS1-Mediated Glutaminolysis Unbridled by MALT1 Protease Promotes Psoriasis Pathogenesis. J. Clin. Investig. 2020, 130, 5180–5196.
- Meng, Z.; Cao, G.; Yang, Q.; Yang, H.; Hao, J.; Yin, Z. Metabolic Control of γδ T Cell Function. Infect. Microbes Dis. 2021, 3, 142–148.
- Hu, Y.-M.; Yeh, C.-L.; Pai, M.-H.; Lee, W.-Y.; Yeh, S.-L. Glutamine Administration Modulates Lung γδ T Lymphocyte Expression in Mice with Polymicrobial Sepsis. Shock 2014, 41, 115–122.
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661.
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219.
- Cooper, A.J.R.; Lalor, S.J.; McLoughlin, R.M. Activation of Human Vδ2+ γδ T Cells by Staphylococcus aureus Promotes Enhanced Anti-Staphylococcal Adaptive Immunity. J. Immunol. 2020, 205, 1039–1049.
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12, 705360.
- Alphonse, M.P.; Rubens, J.H.; Ortines, R.V.; Orlando, N.A.; Patel, A.M.; Dikeman, D.; Wang, Y.; Vuong, I.; Joyce, D.P.; Zhang, J.; et al. Pan-Caspase Inhibition as a Potential Host-Directed Immunotherapy against MRSA and Other Bacterial Skin Infections. Sci. Transl. Med. 2021, 13, eabe9887.
- Cahill, E.; Oladipo, O.O.; Dikeman, D.; Prifti, D.; Mento, S.J.; Miller, L.S.; Alphonse, M.P. An Oral Caspase Inhibitor as Monotherapy or with Antibiotics Eradicates MRSA Skin Infections in Mice. Drug Dev. Res. 2023, 84, 1567–1571.
- Mata Forsberg, M.; Arasa, C.; Van Zwol, W.; Uzunçayır, S.; Schönbichler, A.; Regenthal, P.; Schelin, J.; Lindkvist-Petersson, K.; Björkander, S.; Sverremark-Ekström, E. Activation of Human γδ T Cells and NK Cells by Staphylococcal Enterotoxins Requires Both Monocytes and Conventional T Cells. J. Leukoc. Biol. 2022, 111, 597–609.
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal Enterotoxins. Toxins 2010, 2, 2177–2197.
- Rust, C.J.J.; Verreck, F.; Vietor, H.; Koning, F. Specific Recognition of Staphylococcal Enterotoxin A by Human T Cells Bearing Receptors with the Vγ9 Region. Nature 1990, 346, 572–574.
- Fikri, Y.; Denis, O.; Pastoret, P.-P.; Nyabenda, J. Purified Bovine WC1+ γδ T Lymphocytes Are Activated by Staphylococcal Enterotoxins and Toxic Shock Syndrome Toxin-1 Superantigens: Proliferation Response, TCR Vγ Profile and Cytokines Expression. Immunol. Lett. 2001, 77, 87–95.
- Kumar, S.; Colpitts, S.L.; Ménoret, A.; Budelsky, A.L.; Lefrancois, L.; Vella, A.T. Rapid αβ T-Cell Responses Orchestrate Innate Immunity in Response to Staphylococcal Enterotoxin A. Mucosal Immunol. 2013, 6, 1006–1015.
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus Cutaneous Infections. Nat. Rev. Immunol. 2011, 11, 505–518.
- Zenobia, C.; Hajishengallis, G. Basic Biology and Role of Interleukin-17 in Immunity and Inflammation. Periodontology 2000 2015, 69, 142–159.
- Ménoret, A.; Buturla, J.A.; Xu, M.M.; Svedova, J.; Kumar, S.; Rathinam, V.A.K.; Vella, A.T. T Cell-Directed IL-17 Production by Lung Granular γδ T Cells Is Coordinated by a Novel IL-2 and IL-1β Circuit. Mucosal Immunol. 2018, 11, 1398–1407.
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.; Hennighausen, L.; et al. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity 2007, 26, 371–381.
- Kalyan, S.; Chow, A.W. Human Peripheral γδ T Cells Potentiate the Early Proinflammatory Cytokine Response to Staphylococcal Toxic Shock Syndrome Toxin–1. J. Infect. Dis. 2004, 189, 1892–1896.
- Tkaczyk, C.; Hamilton, M.M.; Datta, V.; Yang, X.P.; Hilliard, J.J.; Stephens, G.L.; Sadowska, A.; Hua, L.; O’Day, T.; Suzich, J.; et al. Staphylococcus aureus Alpha Toxin Suppresses Effective Innate and Adaptive Immune Responses in a Murine Dermonecrosis Model. PLoS ONE 2013, 8, e75103.
- Mölne, L.; Corthay, A.; Holmdahl, R.; Tarkowski, A. Role of Gamma/Delta T Cell Receptor-Expressing Lymphocytes in Cutaneous Infection Caused by Staphylococcus aureus. Clin. Exp. Immunol. 2003, 132, 209–215.
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. IL-17 Is Essential for Host Defense against Cutaneous Staphylococcus aureus Infection in Mice. J. Clin. Investig. 2010, 120, 1762–1773.
- Myles, I.A.; Fontecilla, N.M.; Valdez, P.A.; Vithayathil, P.J.; Naik, S.; Belkaid, Y.; Ouyang, W.; Datta, S.K. Signaling via the IL-20 Receptor Inhibits Cutaneous Production of IL-1β and IL-17A to Promote Infection with Methicillin-Resistant Staphylococcus aureus. Nat. Immunol. 2013, 14, 804–811.
- Hamada, S.; Umemura, M.; Shiono, T.; Tanaka, K.; Yahagi, A.; Begum, M.D.; Oshiro, K.; Okamoto, Y.; Watanabe, H.; Kawakami, K.; et al. IL-17A Produced by Gammadelta T Cells Plays a Critical Role in Innate Immunity against Listeria monocytogenes Infection in the Liver. J. Immunol. 2008, 181, 3456–3463.
- Marchitto, M.C.; Dillen, C.A.; Liu, H.; Miller, R.J.; Archer, N.K.; Ortines, R.V.; Alphonse, M.P.; Marusina, A.I.; Merleev, A.A.; Wang, Y.; et al. Clonal Vγ6 + Vδ4 + T Cells Promote IL-17–Mediated Immunity against Staphylococcus aureus Skin Infection. Proc. Natl. Acad. Sci. USA 2019, 116, 10917–10926.
- Dillen, C.A.; Pinsker, B.L.; Marusina, A.I.; Merleev, A.A.; Farber, O.N.; Liu, H.; Archer, N.K.; Lee, D.B.; Wang, Y.; Ortines, R.V.; et al. Clonally Expanded γδ T Cells Protect against Staphylococcus aureus Skin Reinfection. J. Clin. Investig. 2018, 128, 1026–1042.
- Ishigame, H.; Kakuta, S.; Nagai, T.; Kadoki, M.; Nambu, A.; Komiyama, Y.; Fujikado, N.; Tanahashi, Y.; Akitsu, A.; Kotaki, H.; et al. Differential Roles of Interleukin-17A and -17F in Host Defense against Mucoepithelial Bacterial Infection and Allergic Responses. Immunity 2009, 30, 108–119.
- Wang, Y.; Ahmadi, M.Z.; Dikeman, D.A.; Youn, C.; Archer, N.K. γδ T Cell-Intrinsic IL-1R Promotes Survival during Staphylococcus aureus Bacteremia. Front. Immunol. 2023, 14, 1171934.
- Ramírez-Valle, F.; Gray, E.E.; Cyster, J.G. Inflammation Induces Dermal Vγ4+ γδT17 Memory-like Cells That Travel to Distant Skin and Accelerate Secondary IL-17-Driven Responses. Proc. Natl. Acad. Sci. USA 2015, 112, 8046–8051.
- Christensen, G.J.M.; Brüggemann, H. Bacterial Skin Commensals and Their Role as Host Guardians. Benef. Microbes 2014, 5, 201–215.
- Strbo, N.; Pastar, I.; Romero, L.; Chen, V.; Vujanac, M.; Sawaya, A.P.; Jozic, I.; Ferreira, A.D.F.; Wong, L.L.; Head, C.; et al. Single Cell Analyses Reveal Specific Distribution of Anti-Bacterial Molecule Perforin-2 in Human Skin and Its Modulation by Wounding and Staphylococcus aureus Infection. Exp. Dermatol. 2019, 28, 225–232.
- Pastar, I.; O’Neill, K.; Padula, L.; Head, C.R.; Burgess, J.L.; Chen, V.; Garcia, D.; Stojadinovic, O.; Hower, S.; Plano, G.V.; et al. Staphylococcus Epidermidis Boosts Innate Immune Response by Activation of Gamma Delta T Cells and Induction of Perforin-2 in Human Skin. Front. Immunol. 2020, 11, 550946.
- Johansson, M.A.; Björkander, S.; Mata Forsberg, M.; Qazi, K.R.; Salvany Celades, M.; Bittmann, J.; Eberl, M.; Sverremark-Ekström, E. Probiotic Lactobacilli Modulate Staphylococcus aureus-Induced Activation of Conventional and Unconventional T Cells and NK Cells. Front. Immunol. 2016, 7, 273.
- Pan, N.; Liu, B.; Bao, X.; Zhang, H.; Sheng, S.; Liang, Y.; Pan, H.; Wang, X. Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus aureus Pulmonary and Skin Infections. Vaccines 2021, 9, 984.
- Pan, N.; Liu, Y.; Zhang, H.; Xu, Y.; Bao, X.; Sheng, S.; Liang, Y.; Liu, B.; Lyu, Y.; Li, H.; et al. Oral Vaccination with Engineered Probiotic Limosilactobacillus reuteri Has Protective Effects against Localized and Systemic Staphylococcus aureus Infection. Microbiol. Spectr. 2023, 11, e0367322.
- Cheng, P.; Liu, T.; Zhou, W.-Y.; Zhuang, Y.; Peng, L.; Zhang, J.; Yin, Z.-N.; Mao, X.; Guo, G.; Shi, Y.; et al. Role of Gamma-Delta T Cells in Host Response against Staphylococcus aureus-Induced Pneumonia. BMC Immunol. 2012, 13, 38.
- Baral, P.; Umans, B.D.; Li, L.; Wallrapp, A.; Bist, M.; Kirschbaum, T.; Wei, Y.; Zhou, Y.; Kuchroo, V.K.; Burkett, P.R.; et al. Nociceptor Sensory Neurons Suppress Neutrophil and γδ T Cell Responses in Bacterial Lung Infections and Lethal Pneumonia. Nat. Med. 2018, 24, 417–426.
- Paudel, S.; Ghimire, L.; Jin, L.; Baral, P.; Cai, S.; Jeyaseelan, S. NLRC4 Suppresses IL-17A-Mediated Neutrophil-Dependent Host Defense through Upregulation of IL-18 and Induction of Necroptosis during Gram-Positive Pneumonia. Mucosal Immunol. 2019, 12, 247–257.
- Craven, R.R.; Gao, X.; Allen, I.C.; Gris, D.; Wardenburg, J.B.; McElvania-TeKippe, E.; Ting, J.P.; Duncan, J.A. Staphylococcus aureus α-Hemolysin Activates the NLRP3-Inflammasome in Human and Mouse Monocytic Cells. PLoS ONE 2009, 4, e7446.
- Lin, C.-Y.; Roberts, G.W.; Kift-Morgan, A.; Donovan, K.L.; Topley, N.; Eberl, M. Pathogen-Specific Local Immune Fingerprints Diagnose Bacterial Infection in Peritoneal Dialysis Patients. J. Am. Soc. Nephrol. 2013, 24, 2002–2009.
