Histoplasma capsulatum, the etiological agent for histoplasmosis, is a dimorphic fungus that grows as a mold in the environment and as a yeast in human tissues. It has a broad global distribution with shifting epidemiology. While in immunocompetent individuals infection is usually self-resolving, solid organ transplant recipients are at increased risk of symptomatic disease with dissemination to extrapulmonary tissue. Diagnosis of histoplasmosis relies on direct observation of the pathogen (histopathology, cytopathology, and culture) or detection of antigens, antibodies, or nucleic acids. All transplant recipients with histoplasmosis warrant therapy, though the agent of choice and duration of therapy depends on the severity of disease.
- histoplasmosis
- solid organ transplant
- transplant
- Histoplasma
1. Introduction
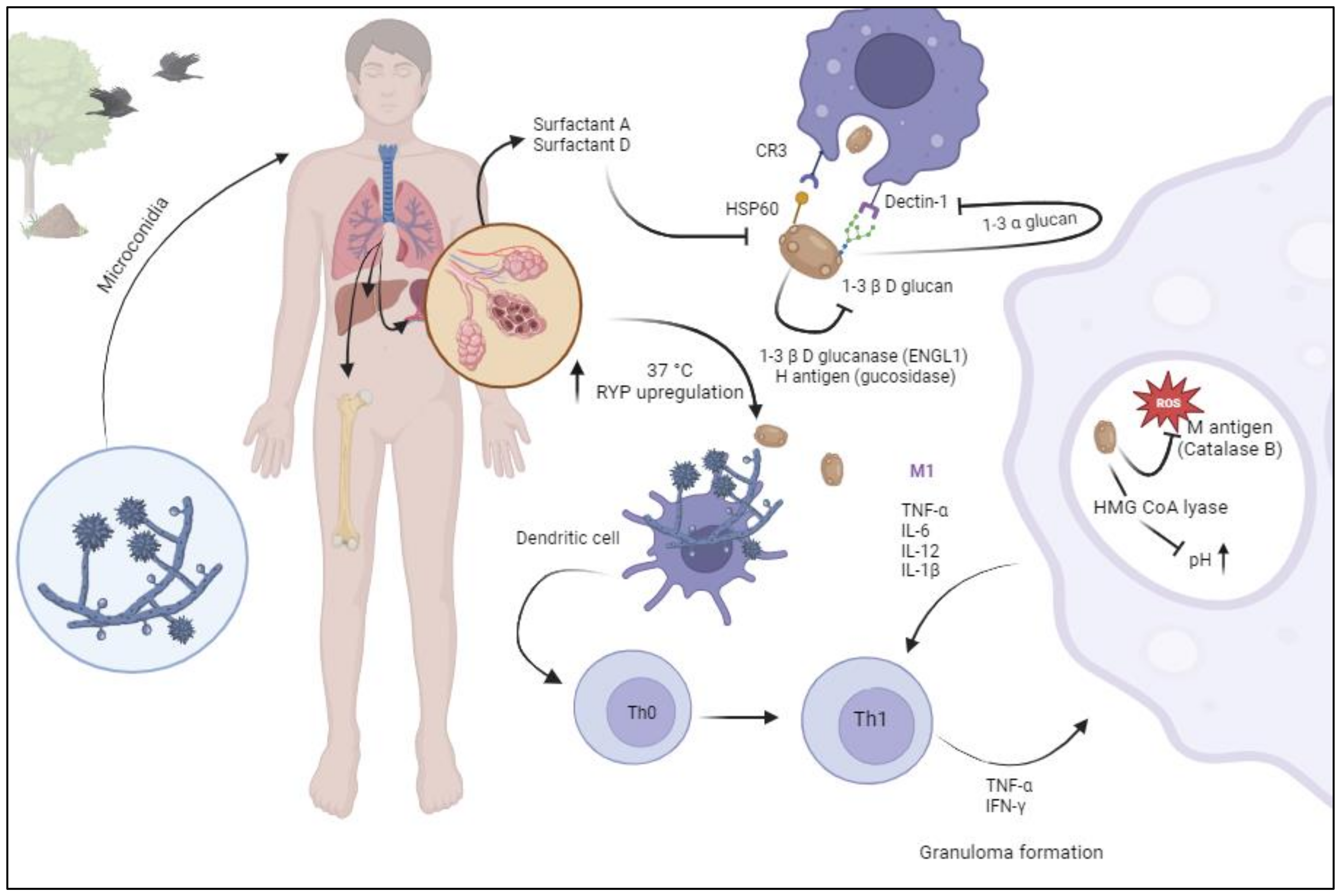
2. Pathogenesis
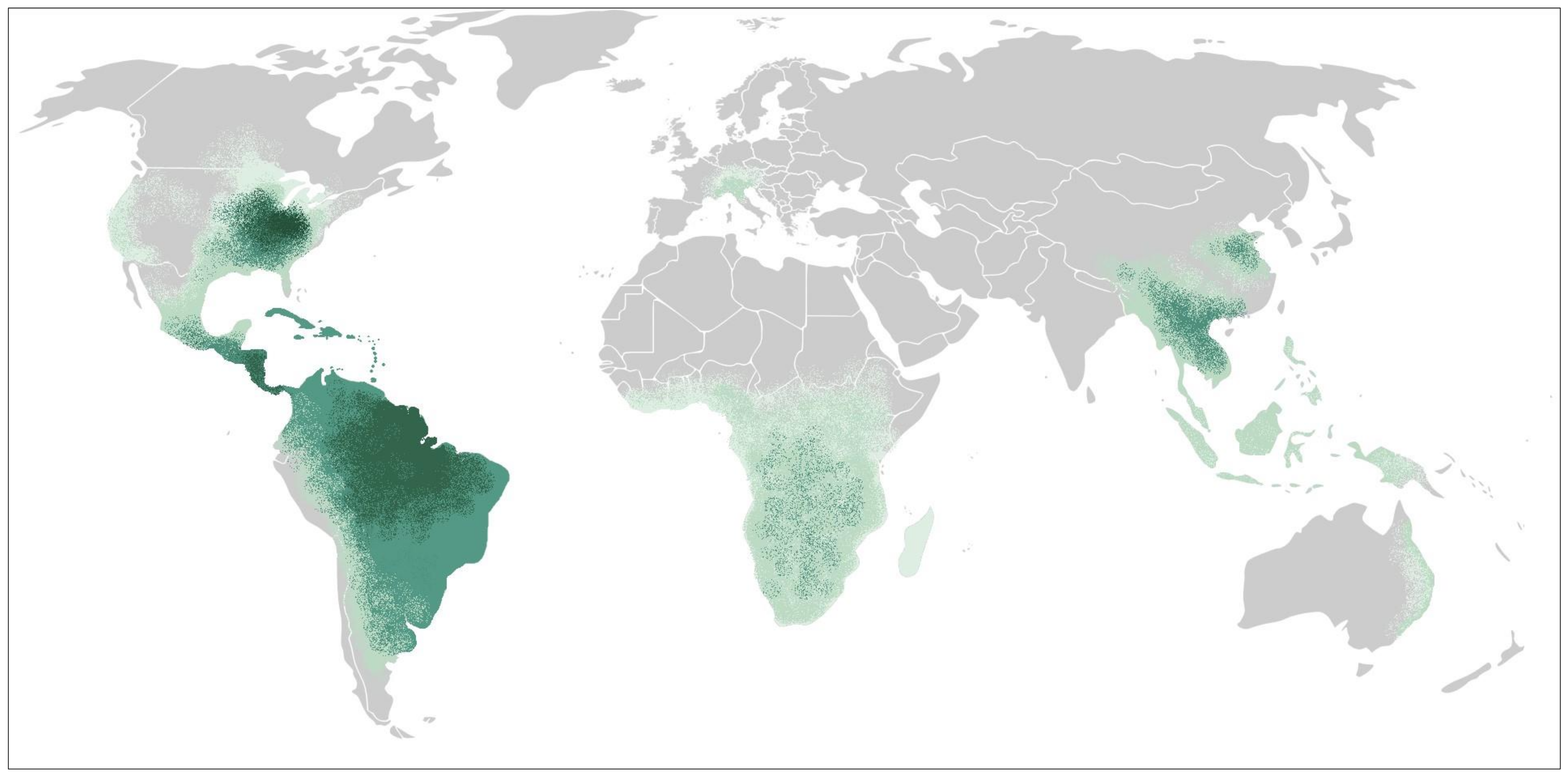
3. Epidemiology and Risk Factors
4. Clinical Presentation
5. Diagnostics
5.1. Culture, Histopathology and Cytopathology
5.2. Antigen
5.3. 1-3-β-D-Glucan
5.4. Aspergillus Galactomannan
5.5. Serology
5.6. Molecular Tests
6. Management
7. Novel Antifungal Therapies and Strategies
8. Peri-Transplant Donor and Recipient Considerations
This entry is adapted from the peer-reviewed paper 10.3390/jof10020124
References
- Teixeira Mde, M.; Patane, J.S.; Taylor, M.L.; Gomez, B.L.; Theodoro, R.C.; de Hoog, S.; Engelthaler, D.M.; Zancope-Oliveira, R.M.; Felipe, M.S.; Barker, B.M. Worldwide Phylogenetic Distributions and Population Dynamics of the Genus Histoplasma. PLoS Negl. Trop. Dis. 2016, 10, e0004732.
- Ocansey, B.K.; Kosmidis, C.; Agyei, M.; Dorkenoo, A.M.; Ayanlowo, O.O.; Oladele, R.O.; Darre, T.; Denning, D.W. Histoplasmosis in Africa: Current perspectives, knowledge gaps, and research priorities. PLoS Negl. Trop. Dis. 2022, 16, e0010111.
- Sepulveda, V.E.; Marquez, R.; Turissini, D.A.; Goldman, W.E.; Matute, D.R. Genome Sequences Reveal Cryptic Speciation in the Human Pathogen Histoplasma capsulatum. mBio 2017, 8.
- Baddley, J.W.; Winthrop, K.L.; Patkar, N.M.; Delzell, E.; Beukelman, T.; Xie, F.; Chen, L.; Curtis, J.R. Geographic distribution of endemic fungal infections among older persons, United States. Emerg. Infect. Dis. 2011, 17, 1664–1669.
- Edwards, L.B.; Acquaviva, F.A.; Livesay, V.T.; Cross, F.W.; Palmer, C.E. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am. Rev. Respir. Dis. 1969, 99, 1–132.
- Vail, G.M.; Young, R.S.; Wheat, L.J.; Filo, R.S.; Cornetta, K.; Goldman, M. Incidence of histoplasmosis following allogeneic bone marrow transplant or solid organ transplant in a hyperendemic area. Transpl. Infect. Dis. 2002, 4, 148–151.
- Bahr, N.C.; Antinori, S.; Wheat, L.J.; Sarosi, G.A. Histoplasmosis infections worldwide: Thinking outside of the Ohio River valley. Curr. Trop. Med. Rep. 2015, 2, 70–80.
- Mazi, P.B.; Sahrmann, J.M.; Olsen, M.A.; Coler-Reilly, A.; Rauseo, A.M.; Pullen, M.; Zuniga-Moya, J.C.; Powderly, W.G.; Spec, A. The Geographic Distribution of Dimorphic Mycoses in the United States for the Modern Era. Clin. Infect. Dis. 2023, 76, 1295–1301.
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppie, P.; Chiller, T.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis. 2018, 18, 1150–1159.
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865.
- Azar, M.M.; Loyd, J.L.; Relich, R.F.; Wheat, L.J.; Hage, C.A. Current Concepts in the Epidemiology, Diagnosis, and Management of Histoplasmosis Syndromes. Semin. Respir. Crit. Care Med. 2020, 41, 13–30.
- Benedict, K.; Mody, R.K. Epidemiology of Histoplasmosis Outbreaks, United States, 1938–2013. Emerg. Infect. Dis. 2016, 22, 370–378.
- Assi, M.; Martin, S.; Wheat, L.J.; Hage, C.; Freifeld, A.; Avery, R.; Baddley, J.W.; Vergidis, P.; Miller, R.; Andes, D.; et al. Histoplasmosis after solid organ transplant. Clin. Infect. Dis. 2013, 57, 1542–1549.
- Colombo, A.L.; Tobon, A.; Restrepo, A.; Queiroz-Telles, F.; Nucci, M. Epidemiology of endemic systemic fungal infections in Latin America. Med. Mycol. 2011, 49, 785–798.
- Maiga, A.W.; Deppen, S.; Scaffidi, B.K.; Baddley, J.; Aldrich, M.C.; Dittus, R.S.; Grogan, E.L. Mapping Histoplasma capsulatum Exposure, United States. Emerg. Infect. Dis. 2018, 24, 1835–1839.
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227.
- Beyhan, S.; Gutierrez, M.; Voorhies, M.; Sil, A. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 2013, 11, e1001614.
- McCormack, F.X.; Gibbons, R.; Ward, S.R.; Kuzmenko, A.; Wu, H.; Deepe, G.S., Jr. Macrophage-independent fungicidal action of the pulmonary collectins. J. Biol. Chem. 2003, 278, 36250–36256.
- Carreto-Binaghi, L.E.; Tenorio, E.P.; Morales-Villarreal, F.R.; Aliouat, E.M.; Zenteno, E.; Martinez-Orozco, J.A.; Taylor, M.L. Detection of Cytokines and Collectins in Bronchoalveolar Fluid Samples of Patients Infected with Histoplasma capsulatum and Pneumocystis jirovecii. J. Fungi 2021, 7, 938.
- Ericson, P.A.; Mirgorodskaya, E.; Hammar, O.S.; Viklund, E.A.; Almstrand, A.R.; Larsson, P.J.; Riise, G.C.; Olin, A.C. Low Levels of Exhaled Surfactant Protein A Associated With BOS After Lung Transplantation. Transplant. Direct 2016, 2, e103.
- Kimberg, M.; Brown, G.D. Dectin-1 and its role in antifungal immunity. Med. Mycol. 2008, 46, 631–636.
- Huang, J.H.; Lin, C.Y.; Wu, S.Y.; Chen, W.Y.; Chu, C.L.; Brown, G.D.; Chuu, C.P.; Wu-Hsieh, B.A. CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway. PLoS Pathog. 2015, 11, e1004985.
- Rappleye, C.A.; Eissenberg, L.G.; Goldman, W.E. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 1366–1370.
- Garfoot, A.L.; Shen, Q.; Wuthrich, M.; Klein, B.S.; Rappleye, C.A. The Eng1 beta-Glucanase Enhances Histoplasma Virulence by Reducing beta-Glucan Exposure. mBio 2016, 7, e01388-15.
- Fisher, K.L.; Woods, J.P. Determination of beta-glucosidase enzymatic function of the Histoplasma capsulatum H antigen using a native expression system. Gene 2000, 247, 191–197.
- Valdez, A.F.; Miranda, D.Z.; Guimaraes, A.J.; Nimrichter, L.; Nosanchuk, J.D. Pathogenicity & virulence of Histoplasma capsulatum—A multifaceted organism adapted to intracellular environments. Virulence 2022, 13, 1900–1919.
- Nittler, M.P.; Hocking-Murray, D.; Foo, C.K.; Sil, A. Identification of Histoplasma capsulatum transcripts induced in response to reactive nitrogen species. Mol. Biol. Cell 2005, 16, 4792–4813.
- Holbrook, E.D.; Smolnycki, K.A.; Youseff, B.H.; Rappleye, C.A. Redundant catalases detoxify phagocyte reactive oxygen and facilitate Histoplasma capsulatum pathogenesis. Infect. Immun. 2013, 81, 2334–2346.
- Youseff, B.H.; Holbrook, E.D.; Smolnycki, K.A.; Rappleye, C.A. Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PLoS Pathog. 2012, 8, e1002713.
- Isaac, D.T.; Coady, A.; Van Prooyen, N.; Sil, A. The 3-hydroxy-methylglutaryl coenzyme A lyase HCL1 is required for macrophage colonization by human fungal pathogen Histoplasma capsulatum. Infect. Immun. 2013, 81, 411–420.
- Heninger, E.; Hogan, L.H.; Karman, J.; Macvilay, S.; Hill, B.; Woods, J.P.; Sandor, M. Characterization of the Histoplasma capsulatum-induced granuloma. J. Immunol. 2006, 177, 3303–3313.
- Gildea, L.A.; Morris, R.E.; Newman, S.L. Histoplasma capsulatum yeasts are phagocytosed via very late antigen-5, killed, and processed for antigen presentation by human dendritic cells. J. Immunol. 2001, 166, 1049–1056.
- McDermott, A.J.; Klein, B.S. Helper T-cell responses and pulmonary fungal infections. Immunology 2018, 155, 155–163.
- Vergidis, P.; Avery, R.K.; Wheat, L.J.; Dotson, J.L.; Assi, M.A.; Antoun, S.A.; Hamoud, K.A.; Burdette, S.D.; Freifeld, A.G.; McKinsey, D.S.; et al. Histoplasmosis complicating tumor necrosis factor-alpha blocker therapy: A retrospective analysis of 98 cases. Clin. Infect. Dis. 2015, 61, 409–417.
- Schwarz, J.; Furcolow, M.L. Some epidemiologic factors and diagnostic tests in blastomycosis, coccidioidomycosis and histoplasmosis. Am. J. Clin. Pathol. 1955, 25, 261–265.
- Benedict, K.; Toda, M.; Jackson, B.R. Revising Conventional Wisdom About Histoplasmosis in the United States. Open Forum Infect. Dis. 2021, 8, ofab306.
- Saullo, J.L.; Miller, R.A. Updates on Histoplasmosis in Solid Organ Transplantation. Curr. Fungal Infect. Rep. 2022, 16, 165–178.
- Hage, C.; Kleiman, M.B.; Wheat, L.J. Histoplasmosis in solid organ transplant recipients. Clin. Infect. Dis. 2010, 50, 122–123; author reply 123–124.
- Brunet, K.; Alanio, A.; Lortholary, O.; Rammaert, B. Reactivation of dormant/latent fungal infection. J. Infect. 2018, 77, 463–468.
- Benedict, K.; McCracken, S.; Signs, K.; Ireland, M.; Amburgey, V.; Serrano, J.A.; Christophe, N.; Gibbons-Burgener, S.; Hallyburton, S.; Warren, K.A.; et al. Enhanced Surveillance for Histoplasmosis-9 States, 2018–2019. Open Forum Infect. Dis. 2020, 7, ofaa343.
- Cuellar-Rodriguez, J.; Avery, R.K.; Lard, M.; Budev, M.; Gordon, S.M.; Shrestha, N.K.; van Duin, D.; Oethinger, M.; Mawhorter, S.D. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin. Infect. Dis. 2009, 49, 710–716.
- Freifeld, A.G.; Iwen, P.C.; Lesiak, B.L.; Gilroy, R.K.; Stevens, R.B.; Kalil, A.C. Histoplasmosis in solid organ transplant recipients at a large Midwestern university transplant center. Transpl. Infect. Dis. 2005, 7, 109–115.
- Benedict, K.; Derado, G.; Mody, R.K. Histoplasmosis-Associated Hospitalizations in the United States, 2001–2012. Open Forum Infect. Dis. 2016, 3, ofv219.
- Batista, M.V.; Pierrotti, L.C.; Abdala, E.; Clemente, W.T.; Girao, E.S.; Rosa, D.R.; Ianhez, L.E.; Bonazzi, P.R.; Lima, A.S.; Fernandes, P.F.; et al. Endemic and opportunistic infections in Brazilian solid organ transplant recipients. Trop. Med. Int. Health 2011, 16, 1134–1142.
- Guimaraes, L.F.; Halpern, M.; de Lemos, A.S.; de Gouvea, E.F.; Goncalves, R.T.; da Rosa Santos, M.A.; Nucci, M.; Santoro-Lopes, G. Invasive Fungal Disease in Renal Transplant Recipients at a Brazilian Center: Local Epidemiology Matters. Transplant. Proc. 2016, 48, 2306–2309.
- Reis, M.A.; Costa, R.S.; Ferraz, A.S. Causes of death in renal transplant recipients: A study of 102 autopsies from 1968 to 1991. J. R. Soc. Med. 1995, 88, 24–27.
- Barros, N.; Wheat, J.L.; Hage, C. Pulmonary Histoplasmosis: A Clinical Update. J. Fungi 2023, 9, 236.
- Grim, S.A.; Proia, L.; Miller, R.; Alhyraba, M.; Costas-Chavarri, A.; Oberholzer, J.; Clark, N.M. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl. Infect. Dis. 2012, 14, 17–23.
- Sethi, J.; Gupta, K.L.; Mohanty, T.; Gupta, S.; Ahluwalia, J.; Kohli, H.S. Fever of Unknown Origin in a Renal Transplant Recipient: Lactate Dehydrogenase as an Important Clue to Diagnosis. Exp. Clin. Transplant. 2020, 18, 390–391.
- Luckett, K.; Dummer, J.S.; Miller, G.; Hester, S.; Thomas, L. Histoplasmosis in Patients With Cell-Mediated Immunodeficiency: Human Immunodeficiency Virus Infection, Organ Transplantation, and Tumor Necrosis Factor-alpha Inhibition. Open Forum Infect. Dis. 2015, 2, ofu116.
- Washburn, L.; Galvan, N.T.; Dhingra, S.; Rana, A.; Goss, J.A. Histoplasmosis hepatitis after orthotopic liver transplantation. J. Surg. Case Rep. 2017, 2017, rjx232.
- Giordani, M.C.; Villamil Cortez, S.K.; Diehl, M.; Barcan, L.A.; Rosa-Diez, G.; Groppa, S.R.; Schreck, C.; Mombelli, C.; Imperiali, N. Hypercalcemia as an Early Finding of Opportunistic Fungal Pneumonia in Renal Transplantation: A Case Series Report. Transplant. Proc. 2020, 52, 1178–1182.
- Jagadish, I.; Chen, W.J.; Agarwal, R.; Shoela, R. Case Report of Disseminated Adrenal Histoplasmosis and Secondary Adrenal Insufficiency. Cureus 2022, 14, e30614.
- Kauffman, C.A. Histoplasmosis: A clinical and laboratory update. Clin. Microbiol. Rev. 2007, 20, 115–132.
- Townsend, J.L.; Shanbhag, S.; Hancock, J.; Bowman, K.; Nijhawan, A.E. Histoplasmosis-Induced Hemophagocytic Syndrome: A Case Series and Review of the Literature. Open Forum Infect. Dis. 2015, 2, ofv055.
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821.
- Connolly, P.A.; Durkin, M.M.; Lemonte, A.M.; Hackett, E.J.; Wheat, L.J. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 2007, 14, 1587–1591.
- Torres-Gonzalez, P.; Niembro-Ortega, M.D.; Martinez-Gamboa, A.; Ahumada-Topete, V.H.; Andrade-Villanueva, J.; Araujo-Melendez, J.; Chaparro-Sanchez, A.; Crabtree-Ramirez, B.; Cruz-Martinez, S.; Gamboa-Dominguez, A.; et al. Diagnostic accuracy cohort study and clinical value of the Histoplasma urine antigen (ALPHA Histoplasma EIA) for disseminated histoplasmosis among HIV infected patients: A multicenter study. PLoS Negl. Trop. Dis. 2018, 12, e0006872.
- Martinez-Gamboa, A.; Niembro-Ortega, M.D.; Torres-Gonzalez, P.; Santiago-Cruz, J.; Velazquez-Zavala, N.G.; Rangel-Cordero, A.; Crabtree-Ramirez, B.; Gamboa-Dominguez, A.; Reyes-Gutierrez, E.; Reyes-Teran, G.; et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: A prospective multicenter study in Mexico. PLoS Negl. Trop. Dis. 2021, 15, e0009215.
- Theel, E.S.; Jespersen, D.J.; Harring, J.; Mandrekar, J.; Binnicker, M.J. Evaluation of an enzyme immunoassay for detection of Histoplasma capsulatum antigen from urine specimens. J. Clin. Microbiol. 2013, 51, 3555–3559.
- Theel, E.S.; Harring, J.A.; Dababneh, A.S.; Rollins, L.O.; Bestrom, J.E.; Jespersen, D.J. Reevaluation of commercial reagents for detection of Histoplasma capsulatum antigen in urine. J. Clin. Microbiol. 2015, 53, 1198–1203.
- Zhang, C.; Lei, G.S.; Lee, C.H.; Hage, C.A. Evaluation of two new enzyme immunoassay reagents for diagnosis of histoplasmosis in a cohort of clinically characterized patients. Med. Mycol. 2015, 53, 868–873.
- Krishnan, G.; Power, M.; Bariola, J.R.; Dare, R. Comparison of Indirect Fungal Diagnostic Tests in Patients With Proven Histoplasmosis. Open Forum Infect. Dis. 2022, 9, ofac609.
- Hage, C.A.; Davis, T.E.; Egan, L.; Parker, M.; Fuller, D.; Lemonte, A.M.; Durkin, M.; Connelly, P.; Joseph Wheat, L.; Blue-Hnidy, D.; et al. Diagnosis of pulmonary histoplasmosis and blastomycosis by detection of antigen in bronchoalveolar lavage fluid using an improved second-generation enzyme-linked immunoassay. Respir. Med. 2007, 101, 43–47.
- Bloch, K.C.; Myint, T.; Raymond-Guillen, L.; Hage, C.A.; Davis, T.E.; Wright, P.W.; Chow, F.C.; Woc-Colburn, L.; Khairy, R.N.; Street, A.C.; et al. Improvement in Diagnosis of Histoplasma Meningitis by Combined Testing for Histoplasma Antigen and Immunoglobulin G and Immunoglobulin M Anti-Histoplasma Antibody in Cerebrospinal Fluid. Clin. Infect. Dis. 2018, 66, 89–94.
- Abdallah, W.; Myint, T.; LaRue, R.; Minderman, M.; Gunn, S.; Wheat, L.J.; Hage, C.A. Diagnosis of Histoplasmosis Using the MVista Histoplasma Galactomannan Antigen Qualitative Lateral Flow-Based Immunoassay: A Multicenter Study. Open Forum Infect. Dis. 2021, 8, ofab454.
- Caceres, D.H.; Gomez, B.L.; Tobon, A.M.; Chiller, T.M.; Lindsley, M.D. Evaluation of a Histoplasma antigen lateral flow assay for the rapid diagnosis of progressive disseminated histoplasmosis in Colombian patients with AIDS. Mycoses 2020, 63, 139–144.
- Caceres, D.H.; Gomez, B.L.; Tobon, A.M.; Minderman, M.; Bridges, N.; Chiller, T.; Lindsley, M.D. Validation and Concordance Analysis of a New Lateral Flow Assay for Detection of Histoplasma Antigen in Urine. J. Fungi 2021, 7, 799.
- Caceres, D.H.; Gomez, B.L.; Tobon, A.M.; Chiller, T.M.; Lindsley, M.D. Evaluation of OIDx Histoplasma Urinary Antigen EIA. Mycopathologia 2022, 187, 129–131.
- Ocansey, B.K.; Otoo, B.; Asamoah, I.; Ganu, V.; Berko, K.P.; Oladele, O.; Amankwa, E.A.; Opoku-Asare, B.; Agyei, M.; George, L.; et al. Cryptococcal and Histoplasma Antigen Screening Among People With Human Immunodeficiency Virus in Ghana and Comparative Analysis of OIDx Histoplasma Lateral Flow Assay and IMMY Histoplasma Enzyme Immunoassay. Open Forum Infect. Dis. 2022, 9, ofac277.
- Wheat, J.; Wheat, H.; Connolly, P.; Kleiman, M.; Supparatpinyo, K.; Nelson, K.; Bradsher, R.; Restrepo, A. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 1997, 24, 1169–1171.
- Kuberski, T.; Myers, R.; Wheat, L.J.; Durkin, M.; Connolly, P.; Kubak, B.M.; Bruckner, D.; Pegues, D. Diagnosis of coccidioidomycosis by antigen detection using cross-reaction with a Histoplasma antigen. Clin. Infect. Dis. 2007, 44, e50–e54.
- Hage, C.A.; Ribes, J.A.; Wengenack, N.L.; Baddour, L.M.; Assi, M.; McKinsey, D.S.; Hammoud, K.; Alapat, D.; Babady, N.E.; Parker, M.; et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 2011, 53, 448–454.
- Hage, C.A.; Azar, M.M.; Bahr, N.; Loyd, J.; Wheat, L.J. Histoplasmosis: Up-to-Date Evidence-Based Approach to Diagnosis and Management. Semin. Respir. Crit. Care Med. 2015, 36, 729–745.
- Ghorra, N.; Goushchi, A.; Konopnicki, D.; Libois, A.; Lagrou, K.; Wind, A.; Montesinos, I.; Hallin, M.; Deyi, V.Y.M. Disseminated histoplasmosis diagnosed by cross-reactivity with the Aspergillus galactomannan antigen in an HIV-positive patient. J. Mycol. Med. 2022, 32, 101244.
- Assi, M.; Lakkis, I.E.; Wheat, L.J. Cross-reactivity in the Histoplasma antigen enzyme immunoassay caused by sporotrichosis. Clin. Vaccine Immunol. 2011, 18, 1781–1782.
- Maphanga, T.G.; Naicker, S.D.; Gomez, B.L.; Mhlanga, M.; Mpembe, R.S.; Schwartz, I.S.; Bamford, C.; Nel, J.; Govender, N.P. Cross-reactivity of a Histoplasma capsulatum antigen enzyme immunoassay in urine specimens from persons with emergomycosis in South Africa. Med. Mycol. 2021, 59, 672–682.
- Koo, S.; Bryar, J.M.; Page, J.H.; Baden, L.R.; Marty, F.M. Diagnostic performance of the (1→3)-beta-D-glucan assay for invasive fungal disease. Clin. Infect. Dis. 2009, 49, 1650–1659.
- Pickering, J.W.; Sant, H.W.; Bowles, C.A.; Roberts, W.L.; Woods, G.L. Evaluation of a (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005, 43, 5957–5962.
- Egan, L.; Connolly, P.; Wheat, L.J.; Fuller, D.; Dais, T.E.; Knox, K.S.; Hage, C.A. Histoplasmosis as a cause for a positive Fungitell (1→3)-beta-D-glucan test. Med. Mycol. 2008, 46, 93–95.
- Cadena, J.; Thompson, G.R., 3rd; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434.
- Xavier, M.O.; Pasqualotto, A.C.; Cardoso, I.C.; Severo, L.C. Cross-reactivity of Paracoccidioides brasiliensis, Histoplasma capsulatum, and Cryptococcus species in the commercial Platelia Aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 2009, 16, 132–133.
- Ranque, S.; Pelletier, R.; Michel-Nguyen, A.; Dromer, F. Platelia Aspergillus assay for diagnosis of disseminated histoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 941–943.
- Wheat, L.J.; Hackett, E.; Durkin, M.; Connolly, P.; Petraitiene, R.; Walsh, T.J.; Knox, K.; Hage, C. Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 2007, 14, 638–640.
- Toscanini, M.A.; Nusblat, A.D.; Cuestas, M.L. Diagnosis of histoplasmosis: Current status and perspectives. Appl. Microbiol. Biotechnol. 2021, 105, 1837–1859.
- Azar, M.M.; Hage, C.A. Laboratory Diagnostics for Histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620.
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Herman, K.M.; Hage, C.A.; Fuller, D.; Wheat, L.J. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin. Infect. Dis. 2016, 62, 896–902.
- Wheat, J.; Myint, T.; Guo, Y.; Kemmer, P.; Hage, C.; Terry, C.; Azar, M.M.; Riddell, J.; Ender, P.; Chen, S.; et al. Central nervous system histoplasmosis: Multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine 2018, 97, e0245.
- Padhye, A.A.; Smith, G.; McLaughlin, D.; Standard, P.G.; Kaufman, L. Comparative evaluation of a chemiluminescent DNA probe and an exoantigen test for rapid identification of Histoplasma capsulatum. J. Clin. Microbiol. 1992, 30, 3108–3111.
- Valero, C.; Buitrago, M.J.; Gago, S.; Quiles-Melero, I.; Garcia-Rodriguez, J. A matrix-assisted laser desorption/ionization time of flight mass spectrometry reference database for the identification of Histoplasma capsulatum. Med. Mycol. 2018, 56, 307–314.
- Cosio, T.; Gaziano, R.; Fontana, C.; Pistoia, E.S.; Petruccelli, R.; Favaro, M.; Pica, F.; Minelli, S.; Bossa, M.C.; Altieri, A.; et al. Closing the Gap in Proteomic Identification of Histoplasma capsulatum: A Case Report and Review of Literature. J. Fungi 2023, 9, 1019.
- Vasconcellos, I.; Dalla Lana, D.F.; Pasqualotto, A.C. The Role of Molecular Tests in the Diagnosis of Disseminated Histoplasmosis. J. Fungi 2019, 6, 1.
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. 2019, 14, 319–338.
- Wang, N.; Zhao, C.; Tang, C.; Wang, L. Case Report and Literature Review: Disseminated Histoplasmosis Infection Diagnosed by Metagenomic Next-Generation Sequencing. Infect. Drug Resist. 2022, 15, 4507–4514.
- Wheat, L.J.; Cloud, G.; Johnson, P.C.; Connolly, P.; Goldman, M.; Le Monte, A.; Fuller, D.E.; Davis, T.E.; Hafner, R.; Group, A.C.T.; et al. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob. Agents Chemother. 2001, 45, 2354–2357.
- Johnson, P.C.; Wheat, L.J.; Cloud, G.A.; Goldman, M.; Lancaster, D.; Bamberger, D.M.; Powderly, W.G.; Hafner, R.; Kauffman, C.A.; Dismukes, W.E.; et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann. Intern. Med. 2002, 137, 105–109.
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825.
- LeMonte, A.M.; Washum, K.E.; Smedema, M.L.; Schnizlein-Bick, C.; Kohler, S.M.; Wheat, L.J. Amphotericin B combined with itraconazole or fluconazole for treatment of histoplasmosis. J. Infect. Dis. 2000, 182, 545–550.
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176.
- McCreary, E.K.; Davis, M.R.; Narayanan, N.; Andes, D.R.; Cattaneo, D.; Christian, R.; Lewis, R.E.; Watt, K.M.; Wiederhold, N.P.; Johnson, M.D. Utility of triazole antifungal therapeutic drug monitoring: Insights from the Society of Infectious Diseases Pharmacists: Endorsed by the Mycoses Study Group Education and Research Consortium. Pharmacotherapy 2023, 43, 1043–1050.
- Miller, R.; Assi, M.; The AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplant recipients-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13553.
- Laverdiere, M.; Bow, E.J.; Rotstein, C.; Autmizguine, J.; Broady, R.; Garber, G.; Haider, S.; Hussaini, T.; Husain, S.; Ovetchkine, P.; et al. Therapeutic drug monitoring for triazoles: A needs assessment review and recommendations from a Canadian perspective. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 327–343.
- Cartledge, J.D.; Midgely, J.; Gazzard, B.G. Itraconazole solution: Higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J. Clin. Pathol. 1997, 50, 477–480.
- Firkus, D.; Abu Saleh, O.M.; Enzler, M.J.; Jannetto, P.J.; Mara, K.; Vergidis, P.; Rivera, C.G.; Stevens, R.W. Does metabolite matter? Defining target itraconazole and hydroxy-itraconazole serum concentrations for blastomycosis. Mycoses 2023, 66, 412–419.
- Rauseo, A.M.; Mazi, P.; Lewis, P.; Burnett, B.; Mudge, S.; Spec, A. Bioavailability of Single-Dose SUBA-Itraconazole Compared to Conventional Itraconazole under Fasted and Fed Conditions. Antimicrob. Agents Chemother. 2021, 65, e0013421.
- Gupta, A.O.; Singh, N. Immune reconstitution syndrome and fungal infections. Curr. Opin. Infect. Dis. 2011, 24, 527–533.
- Jazwinski, A.; Naggie, S.; Perfect, J. Immune reconstitution syndrome in a patient with disseminated histoplasmosis and steroid taper: Maintaining the perfect balance. Mycoses 2011, 54, 270–272.
- World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living with HIV; World Health Organization: Geneva, Switzerland, 2022.
- Jarvis, J.N.; Lawrence, D.S.; Meya, D.B.; Kagimu, E.; Kasibante, J.; Mpoza, E.; Rutakingirwa, M.K.; Ssebambulidde, K.; Tugume, L.; Rhein, J.; et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. N. Engl. J. Med. 2022, 386, 1109–1120.
- Pasqualotto, A.C.; Lana, D.D.; Godoy, C.S.M.; Leitao, T.; Bay, M.B.; Damasceno, L.S.; Soares, R.B.A.; Kist, R.; Silva, L.R.; Wiltgen, D.; et al. Single High Dose of Liposomal Amphotericin B in Human Immunodeficiency Virus/AIDS-Related Disseminated Histoplasmosis: A Randomized Trial. Clin. Infect. Dis. 2023, 77, 1126–1132.
- Boulware, D.R.; Atukunda, M.; Kagimu, E.; Musubire, A.K.; Akampurira, A.; Tugume, L.; Ssebambulidde, K.; Kasibante, J.; Nsangi, L.; Mugabi, T.; et al. Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial. Clin. Infect. Dis. 2023, 77, 1659–1667.
- Singh, N.; Huprikar, S.; Burdette, S.D.; Morris, M.I.; Blair, J.E.; Wheat, L.J.; the American Society of Transplantation, Infectious Diseases Community of Practice, Donor-Derived Fungal Infection Working Group. Donor-derived fungal infections in organ transplant recipients: Guidelines of the American Society of Transplantation, infectious diseases community of practice. Am. J. Transplant. 2012, 12, 2414–2428.
- Abad, C.L.R.; Razonable, R.R. Donor-derived endemic mycoses after solid organ transplantation: A review of reported cases. Clin. Transplant. 2023, 38, e15199.
