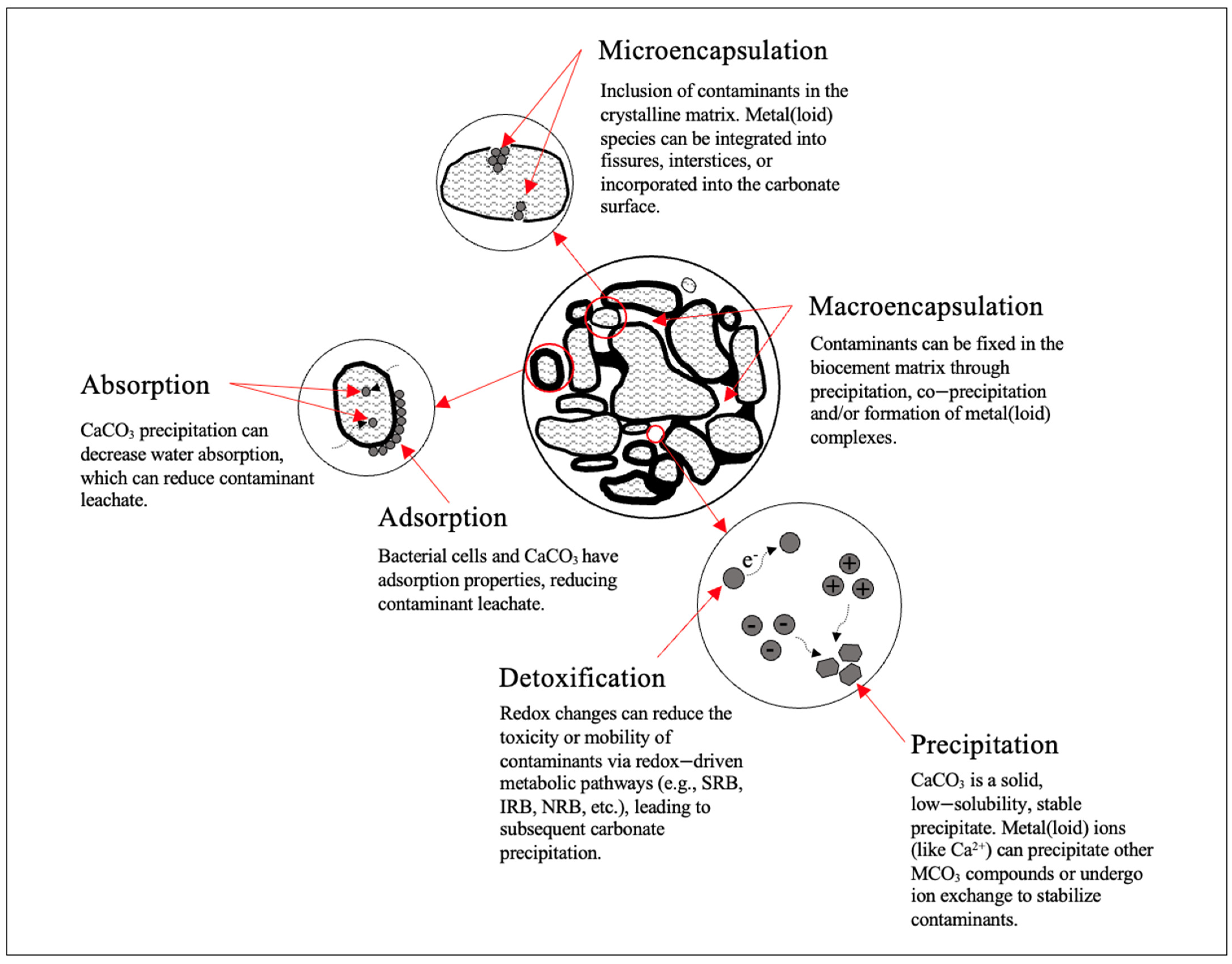
Mining waste represents a global issue due to its potential of generating acidic or alkaline leachate with high concentrations of metals and metalloids (metal(loid)s). Microbial-induced calcium carbonate precipitation (MICP) is an engineering tool used for remediation. MICP, induced via biological activity, aims to precipitate calcium carbonate (CaCO3) or co-precipitate other metal carbonates (MCO3). MICP is a bio-geochemical remediation method that aims to immobilize or remove metal(loid)s via enzyme, redox, or photosynthetic metabolic pathways. Contaminants are removed directly through immobilization as mineral precipitates (CaCO3 or MCO3), or indirectly (via sorption, complexes, or inclusion into the crystal structure). Further, CaCO3 precipitates deposited on the surface or within the pore spaces of a solid matrix create a clogging effect to reduce contaminant leachate. Experimental research on MICP has shown its promise as a bioremediation technique for mining waste.
- mining waste
- bioremediation
- MICP
- precipitation
1. Introduction
2. Biochemical Processes
2.1. MICP
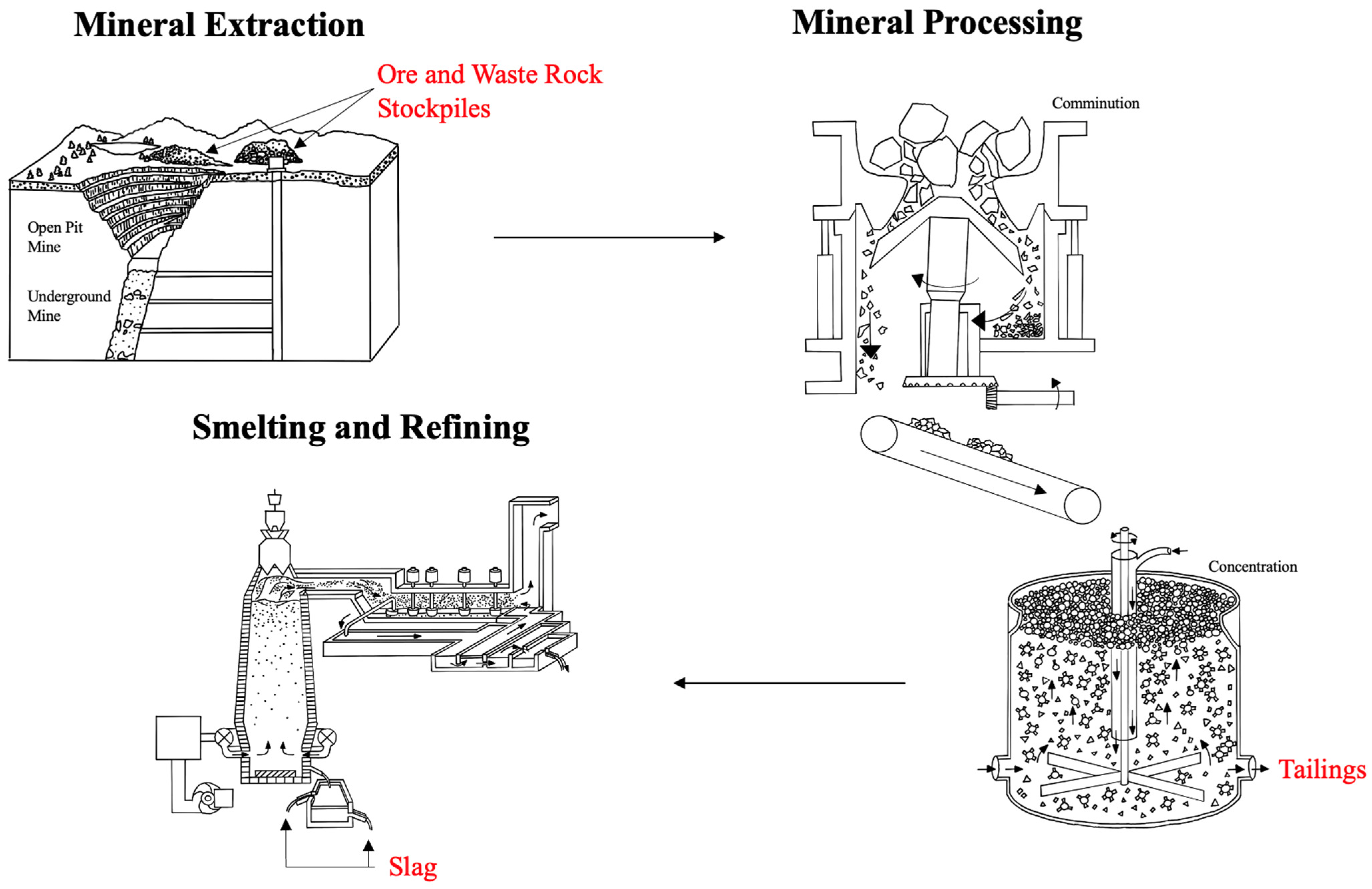
2.2. Mining Waste Characterization and Treatment
3. Bioremediation Processes—Geophysical and Biochemical Interactions
3.1. Bioremediation
This entry is adapted from the peer-reviewed paper 10.3390/toxics12020107
References
- Hamrin, H. Underground Mining Methods and Applications; Atlas Copco: Stockholm, Sweden, 1997; pp. 3–14.
- Encyclopædia Britannica, Inc. A Zinc-Lead Blast Furnace and Lead-Splash Condenser. Available online: https://www.britannica.com/technology/lead-processing (accessed on 21 December 2023).
- Encyclopædia Britannica, Inc. Schematic Diagram of a Flotation Separation Cell. Available online: https://www.britannica.com/technology/mineral-processing/Concentration (accessed on 21 December 2023).
- saVRee Gyratory Crusher Operation. Available online: https://savree.com/en/encyclopedia/gyratory-crusher (accessed on 21 December 2023).
- Santini, T.C.; Banning, N.C. Alkaline Tailings as Novel Soil Forming Substrates: Reframing Perspectives on Mining and Refining Wastes. Hydrometallurgy 2016, 164, 38–47.
- Karpiński, P.H.; Bałdyga, J. Precipitation Processes (Chapter 8). In Handbook of Industrial Crystallization; Myerson, A.S., Erdemir, D., Lee, A.Y., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 216–265. ISBN 978-1-139-02694-9.
- Lewis, A. Precipitation of Heavy Metals. In Sustainable Heavy Metal Remediation: Volume 1: Principles and Processes; Rene, E.R., Sahinkaya, E., Lewis, A., Lens, P.N.L., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2017; pp. 101–120. ISBN 978-3-319-58622-9.
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial Resistance to Metals in the Environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207.
- Diels, L.; Geets, J.; Dejonghe, W.; Roy, S.V.; Vanbroekhoven, K.; Szewczyk, A.; Malina, G. Heavy Metal Immobilization in Groundwater by In Situ Bioprecipitation: Comments and Questions about Efficiency and Sustainability of the Process. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy, Amherst, MA, USA, 16–19 October 2006; The Berkeley Electronic Press: Berkeley, CA, USA, 2010; Volume 11, pp. 99–112.
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of Calcium Carbonate Minerals by Bacteria and Its Multiple Applications. SpringerPlus 2016, 5, 250.
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 1–15.
- Jeyakumar, P.; Debnath, C.; Vijayaraghavan, R.; Muthuraj, M. Trends in Bioremediation of Heavy Metal Contaminations. Environ. Eng. Res. 2023, 28, 1–19.
- Joshi, S.; Goyal, S.; Sudhakara Reddy, M. Influence of Biogenic Treatment in Improving the Durability Properties of Waste Amended Concrete: A Review. Constr. Build. Mater. 2020, 263, 120170.
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29.
- Kaur, G.; Dhami, N.K.; Goyal, S.; Mukherjee, A.; Reddy, M.S. Utilization of Carbon Dioxide as an Alternative to Urea in Biocementation. Constr. Build. Mater. 2016, 123, 527–533.
- Kumar, A.; Song, H.-W.; Mishra, S.; Zhang, W.; Zhang, Y.-L.; Zhang, Q.-R.; Yu, Z.-G. Application of Microbial-Induced Carbonate Precipitation (MICP) Techniques to Remove Heavy Metal in the Natural Environment: A Critical Review. Chemosphere 2023, 318, 137894.
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 1–21.
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization Based Remediation of As(III) Contaminated Soil by Sporosarcina Ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184.
- Smith, L.A.; Alleman, B.C.; Copley-Graves, L. Biological Treatment Options. In Emerging Technology for Bioremediation of Metals; Means, J.L., Hinchee, R.E., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 1–12.
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Férard, C. Significance, Mechanisms and Environmental Implications of Microbial Biomineralization. C. R. Geosci. 2011, 343, 160–167.
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline Residues and the Environment: A Review of Impacts, Management Practices and Opportunities. J. Clean. Prod. 2016, 112, 3571–3582.
- Johnson, D.B.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Sci. Total Environ. 2005, 338, 3–14.
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A Critical Review on Remediation, Reuse, and Resource Recovery from Acid Mine Drainage. Environ. Pollut. 2019, 247, 1110–1124.
- Taylor, J.; Pape, S.; Murphy, N. A Summary of Passive and Active Treatment Technologies for Acid and Metalliferous Drainage (AMD); Australian Centre for Minerals Extension and Research (ACMER): Fremantle, Australia, 2005; p. 49.
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281.
- Khudhur, F.W.K.; MacDonald, J.M.; Macente, A.; Daly, L. The Utilization of Alkaline Wastes in Passive Carbon Capture and Sequestration: Promises, Challenges and Environmental Aspects. Sci. Total Environ. 2022, 823, 153553.
- Gravogl, G.; Birkelbach, F.; Müller, D.; Lengauer, C.L.; Weinberger, P.; Miletich, R. Pressure Dependence of the Low Temperature Carbonation Kinetics of Calcium Oxide for Potential Thermochemical Energy Storage Purposes and Sustainable CO2 Fixation. Adv. Sustain. Syst. 2021, 5, 2100022.
- Pan, S.-Y.; Chang, E.E.; Chiang, P.-C. CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications. Aerosol Air Qual. Res. 2012, 12, 770–791.
- Mujah, D.; Shahin, M.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiology 2016, 34, 524–537.
- Zúñiga-Barra, H.; Toledo-Alarcón, J.; Torres-Aravena, Á.; Jorquera, L.; Rivas, M.; Gutiérrez, L.; Jeison, D. Improving the Sustainable Management of Mining Tailings through Microbially Induced Calcite Precipitation: A Review. Miner. Eng. 2022, 189, 107855.
- Achal, V.; Pan, X.; Lee, D.-J.; Kumari, D.; Zhang, D. Remediation of Cr(VI) from Chromium Slag by Biocementation. Chemosphere 2013, 93, 1352–1358.
- Zhu, X.; Li, W.; Zhan, L.; Huang, M.; Zhang, Q.; Achal, V. The Large-Scale Process of Microbial Carbonate Precipitation for Nickel Remediation from an Industrial Soil. Environ. Pollut. 2016, 219, 149–155.
- Govarthanan, M.; Lee, K.-J.; Cho, M.; Kim, J.S.; Kamala-Kannan, S.; Oh, B.-T. Significance of Autochthonous Bacillus Sp. KK1 on Biomineralization of Lead in Mine Tailings. Chemosphere 2013, 90, 2267–2272.
- Kumari, D.; Pan, X.; Lee, D.-J.; Achal, V. Immobilization of Cadmium in Soil by Microbially Induced Carbonate Precipitation with Exiguobacterium Undae at Low Temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102.
- Proudfoot, D.; Brooks, L.; Gammons, C.H.; Barth, E.; Bless, D.; Nagisetty, R.M.; Lauchnor, E.G. Investigating the Potential for Microbially Induced Carbonate Precipitation to Treat Mine Waste. J. Hazard. Mater. 2022, 424, 127490.
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Ito, M.; Sato, T.; Igarashi, T.; Banda, K.; Chirwa, M.; Nyambe, I.; Nakayama, S.; et al. Efficacy of Biocementation of Lead Mine Waste from the Kabwe Mine Site Evaluated Using Pararhodobacter Sp. Environ. Sci. Pollut. Res. Int. 2019, 26, 15653–15664.
- Kang, C.-H.; Oh, S.J.; Shin, Y.; Han, S.-H.; Nam, I.-H.; So, J.-S. Bioremediation of Lead by Ureolytic Bacteria Isolated from Soil at Abandoned Metal Mines in South Korea. Ecol. Eng. 2015, 74, 402–407.
- Kang, C.-H.; Han, S.-H.; Shin, Y.; Oh, S.J.; So, J.-S. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl. Biochem. Biotechnol. 2014, 172, 2907–2915.
- Achal, V.; Pan, X.; Zhang, D. Bioremediation of Strontium (Sr) Contaminated Aquifer Quartz Sand Based on Carbonate Precipitation Induced by Sr Resistant Halomonas sp. Chemosphere 2012, 89, 764–768.
- Achal, V.; Pan, X.; Zhang, D.; Fu, Q. Bioremediation of Pb-Contaminated Soil Based on Microbially Induced Calcite Precipitation. J. Microbiol. Biotechnol. 2012, 22, 244–247.
- Achal, V.; Pan, X.; Zhang, D. Remediation of Copper-Contaminated Soil by Kocuria Flava CR1, Based on Microbially Induced Calcite Precipitation. Ecol. Eng. 2011, 37, 1601–1605.
- Yavuz, O.; Guzel, R.; Aydin, F.; Tegin, I.; Ziyadanogullari, R. Removal of Cadmium and Lead from Aqueous Solution by Calcite. Pol. J. Environ. Stud. 2007, 16, 467–471.
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy Metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) Removal from Water in Malaysia: Post Treatment by High Quality Limestone. Bioresour. Technol. 2008, 99, 1578–1583.
- LaGrega, M.D.; Buckingham, P.; Evans, J. Hazardous Waste Management, 2nd ed.; Waveland Press, Inc.: Long Grove, IL, USA, 1994.
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial Carbonate Precipitation Improves the Durability of Cementitious Materials. Cem. Concr. Res. 2008, 38, 1005–1014.
- Lai, Y.; Yu, J.; Liu, S.; Liu, J.; Wang, R.; Dong, B. Experimental Study to Improve the Mechanical Properties of Iron Tailings Sand by Using MICP at Low pH. Constr. Build. Mater. 2021, 273, 121729.
- Achal, V.; Pan, X.; Özyurt, N. Improved Strength and Durability of Fly Ash-Amended Concrete by Microbial Calcite Precipitation. Ecol. Eng. 2011, 37, 554–559.
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially Induced Carbonate Precipitation (MICP) for Soil Strengthening: A Comprehensive Review. Biogeotechnics 2023, 1, 100002.
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203.
- Jiang, N.-J.; Wang, Y.-J.; Chu, J.; Kawasaki, S.; Tang, C.-S.; Cheng, L.; Du, Y.-J.; Shashank, B.S.; Singh, D.N.; Han, X.-L.; et al. Bio-Mediated Soil Improvement: An Introspection into Processes, Materials, Characterization and Applications. Soil Use Manag. 2022, 38, 68–93.
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical Processes and Geotechnical Applications: Progress, Opportunities and Challenges. Géotechnique 2013, 63, 287–301.
- Xiangliang, P. Micrologically Induced Carbonate Precipitation as a Promising Way to in Situ Immobilize Heavy Metals in Groundwater and Sediment. Res. J. Chem. Environ. 2009, 13, 3–4.
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Chapter 13—Stabilization/Solidification of Mining Waste via Biocementation. In Low Carbon Stabilization and Solidification of Hazardous Wastes; Tsang, D.C.W., Wang, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–209. ISBN 978-0-12-824004-5.
