Lead–acid battery technology has been effectively fulfilling a variety of energy needs, ranging from classic car industry requirements to current plug-in hybrid electric vehicle requirements through any stationary system. Depending on the operating conditions, the battery can be affected in many ways. The same deterioration mechanisms affect all types of lead–acid batteries but to varying degrees. Two electrodes with the aqueous H2SO4 electrolyte (sulfuric acid) and the terminals are the main components of a lead–acid battery. A grid and the active material—PbO2 as the positive active material and Pb as the negative active material—make up the electrodes.
1. Corrosion
The creation and accumulation of a passive corrosion layer at the interface between the active material and the grid material of the positive plate is one of the key faults in LAB technology. This process has a substantial impact on battery functioning since the electrons created at the positive active material must overcome higher resistances to pass through the positive grid material and reach the external circuit [
38]. Creep, stress corrosion and electrochemical attack all occur as the grid ages. Specially, corrosion accelerates the deterioration of the grid, making the positive plate expand in size. On the other hand, corrosion causes the grid latticework to weaken or disintegrate, eventually preventing the plate from transporting current to the load [
42]. This is due to the internal resistance, which is affected by corrosion. As the corrosion layer thickens, the internal resistance rises; also, available capacity is influenced by corrosion [
43]. Corrosion is due to a number of causes, but the three most important are battery voltage, the concentration of acid and temperature. Generally, increased voltages and higher acid concentrations accelerate corrosion significantly. High temperatures also play for an important role in corrosion: the greater the temperature is, the faster the corrosion is [
42,
43]. This affects the lead–acid battery’s charge/discharge mechanism [
44]. In general, two forms of LAB corrosion have been recorded in the literature [
45]:
-
The growth of a PbO layer between the alloy and the AM in deep discharge circumstances. Because of PbO’s weak electrical conductivity, recharging the active mass is difficult if not impossible in some situations.
-
The oxidation of lead into PbO2 at high anodic potential: This type of corrosion causes the irreversible oxidation of metal by generating enormous pits, which can cause the grids to break mechanically. This phenomenon happens during overcharging, particularly during high-current charging, which is sometimes known as boost charging.
With grids composed of lead–calcium alloys, a special issue occurs: namely, batteries might experience a fast walk down in deep-discharge capacity during cycling. This performance loss is more severe than that seen in batteries with lead–antimony alloys having > 1 wt percent
SbSb and has been dubbed the antimony-free effect [
46]. The mechanical characteristics, micro-structure, electrochemical behavior of active materials and corrosion layers on the
Pb–
Sb electrode are all affected by the antimony concentration [
47,
48]. Also, corrosion changes the current/voltage properties of the battery [
49].
2. Non-Cohesion of Active Material
A prominent aging component in batteries subjected to cycle regimes is the loss of cohesion between individual particles of the PAM. Softening and shedding faults create the non-cohesion of active material; this presents flaws in electronic contact between the AM and the grid [
50,
51]. Because of the difference in substance volume, the conversion of active material
PbO2PbO2 to
PbSO4PbSO4 causes expansion and contraction of positive electrode volume during charging and discharging, resulting in softening and shedding of active substance over time [
52]. Shedding occurs when the active material from the plates falls to the bottom of the battery and gathers. It eventually drives battery cell shorting [
53] if sufficient material builds up at the bottom casing to generate an electrical short. It also can be a result of overcharging the battery (as gassing bubbles can separate the AM from the electrodes), frequent cycling and expansion of the positive plate [
50]. The softening of active material is seen after the battery has been used for a while. It manifests itself as a rapid loss of capacity due to a decrease in AM density below a threshold value. When the AM touches the electrolyte, the ions of the latter flow into the particles and agglomerates, modifying the ratio between the crystal and gel zones and causing the particles to amorphize [
43]. This causes a drop in AM density below a critical value and, as a result, softens the AM, resulting in rapid capacity loss; as a consequence, the battery’s cycle life is reduced [
54].
3. Sulfating of the Electrode
In sulfuric acid, lead sulfate is sparingly soluble and accumulates as particles on active materials during discharge. This sulfate crystal forms on both the negative and positive plates. If the battery is left discharged or undercharged for a long time, it turns into a high-resistance coating. In other words, if the LAB is not working properly, this sulfate grows, and big sulfate crystals are formed [
55,
56]. Because the latter do not dissolve easily during the charging process, irreversible sulfate is formed [
53,
57]. This leads to battery failure because active materials are depleted, and the formation of sulfate increases the battery’s resistance while also reducing the area available for charge transfer processes [
58]. The crystallized lead sulfate not only does not participate in the process, but it also adsorbs on the electrode plate’s surface, increasing the battery’s internal resistance and affecting the battery’s charge and discharge performance as well as capacity [
40,
59]. The consequence is the formation of an insulating electric layer, which slows the acid diffusion. In AGM/VRLA batteries, reversing sulfation is not practical due to the fact that the specified sulfation-reversal voltage is sufficient to motivate out-gassing through the battery’s stress-relief valves [
42,
53], resulting in battery degradation due to electrolyte loss. Sulfated AGM/VRLA batteries need to be replaced [
42]. The results of the effects of sulfation are [
60,
61,
62]:
These effects are also demonstrated in [
39,
62,
63]; the authors of these references investigated the structure and recrystallization of hard crystalline lead sulfate in depth and obtained a good grasp of it using a different experiences; they highlight the following points: Lead sulfate crystals are produced and expand over time when a lead plate with lead oxide on the surface comes into contact with sulfuric acid. Thus, the charge/discharge reactions of the battery do not proceed in the same way, and as a result of the decreased reaction site area per unit volume, the current is reduced.
4. Temperature’s Influence on LABs
A battery’s optimal working temperature is 25 °C. In general, the battery performs best when the electrolyte temperature is kept at a reasonable level [
55]. Temperature has a significant impact on battery aging. LABs have bad low-temperature overall performance due to the rate of the electrochemical reaction of AM and the ionic diffusion velocity decreasing at low temperatures [
43,
55]. Further, low temperatures suppress the electrochemical charge–discharge reaction process, resulting in capacity loss; moreover, this range of temperatures facilitate the formation of an irreversible
PbSO4PbSO4 passivation layer on the negative electrode [
43], leading to irreversible sulfation and battery failure. This irreversible sulfation can be eliminated by high temperatures; however, when the temperature rises, grid corrosion and hydrogen evaporation at the electrodes—causing water loss (overcharging or self-discharge)—also increase. In other words, sulfation occurs when a battery is charged at too low a voltage (undercharging). Excess battery gassing, electrolyte loss and rapid grid corrosion result from charging at too high a voltage (overcharging), deformation and erosion of the plates as well as overheating [
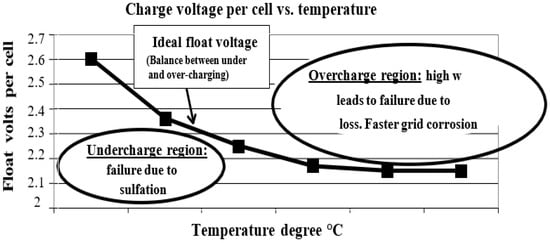
49]. As shown in
Figure 1, excess gassing in AGM/VRLA batteries causes battery dry-out, which leads to failure.
Figure 1. Temperature-compensated charging voltage in a lead–acid starting battery [
42].
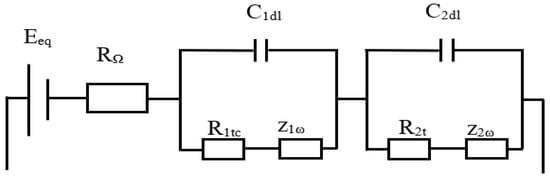
To analyze the impacts of those faults on the electrical components of LABs, the researchers present in Figure 2 a simplified electric circuit of a battery.
Figure 2. Equivalent electrical circuit of a battery [
53].
Where:
-
𝐸𝑒𝑞=𝐸0+−𝐸0− is the total potential of the battery (𝐸0+, 𝐸0− are the potentials of the positive and negative electrodes, respectively). This potential depends on the electrolyte used because it determines the number of electrons that are released when the metal is dissolved. So its variation means that an electrolyte fault has appeared.
-
𝑅𝛺 is the battery’s internal resistance and is the sum of the connector resistances 𝑅1𝑐, 𝑅2𝑐 and the resistance of the electrolyte 𝑅𝑒. Fluctuation of this resistance demonstrates the presence of the stratification or the mechanical degradation of the electrodes.
-
𝐶1𝑑𝑙, 𝐶2𝑑𝑙 is the double-layer capacitance on each electrode. This capacitance is due to a distribution of the charge between the electrode and the electrolyte.
-
𝑅1𝑡𝑐, 𝑅2𝑡𝑐 is the resistance of charge transfer representing the charge transfer phenomenon. Corrosion of the grid occurs as a result of this fluctuation over the battery’s life cycle; more precisely, if 𝑅𝑗𝑡𝑐>𝑅𝑗𝑡𝑐𝑚𝑎𝑥, with 𝑗=1,2.
-
𝑍1𝜔, 𝑍2𝜔 corresponds to the diffusion phenomenon. This is obtained by the concentration degree of the electrolyte close to the electrode. Its deviation from the maximum value (𝑍𝑗𝜔>𝑍𝑗𝜔𝑚𝑎𝑥) expresses that the LAB is sulfated.
During the LAB’s cycle life, obviously these parameters change, including an increase in the internal impedance and an increase in the voltage drop of charge transfer and diffusion during discharge. Because grid corrosion, PAM softening, shedding or sulfation and other faulty states are all linked to battery charging, the charge regime has a significant impact on the cycle life of the LAB [
64].
In addition to lead–acid batteries, the researchers find nickel-based batteries: there are NiMH batteries that have almost replaced NiCd batteries, which have cadmium recycling problems. NiMH batteries have a high energy density and can be completely discharged without affecting their lifespan. They have the disadvantage of being of low capacity, which makes these batteries rather intended to feed portable devices where the autonomy rarely exceeds a few hours.
The third type of batteries is lithium-based, which are divided into:
-
Lithium metal: dangerous and explosive;
-
Lithium ion: stable, with the highest energy density on the market;
-
Lithium polymer: promising dry technology.
Their high discharge capacity (six times better than sealed lead) is their great interest, but their price is still prohibitive.
Due to the different materials and strategies used in each in each of these batteries, they do not share the same faults and the same effects. However, corrosion in lithium batteries—observed in lithium-powder-based electrodes (Lip-electrodes)—affects the delivered capacity of Lip-electrodes and increases the overvoltage of the lithium electro-dissolution process [
65]; this can be observed in the aluminum electrolyte in Li-ion batteries [
58,
66]. This phenomenon in lithium batteries is the result of volume changes that occur during the conversion of hydrogen solid solution to the hydride phase during cycling [
67,
68,
69]. Cobalt, nickel and lithium suffer from sulfate leachate of cathode scrap, which occurs during the processing of Li-ion batteries [
70,
71] and deals with the selective recovery of nickel and lithium from sulfate leachate using extractive separation.
This entry is adapted from the peer-reviewed paper 10.3390/en17051187