Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The industrial sector plays a significant role in global economic growth. However, it also produces polluting effluents that must be treated to prevent environmental damage and ensure the quality of life for future generations is not compromised. Various physical, chemical, and biological methods have been employed to treat industrial effluents. Filamentous fungi, in particular, have garnered attention as effective bioremediation agents due to their ability to produce enzymes capable of degrading recalcitrant compounds, and adsorb different pollutant molecules.
- filamentous fungi
- bioremediation
- industrial effluents
- phenolic compounds
- enzymes
- laccase
1. Introduction
The economic progress and prosperity of a nation is primarily determined by its industrial development. Industries constitute essential elements of modern civilization, supplying vital materials to humankind and creating employment opportunities [1]. Despite the importance of the industrial sector for developing a country, it is essential to note that industries commonly generate a lot of effluent, which can cause environmental damage if discharged into receiving bodies without adequate prior treatment [2].
Industrial effluents are broadly divided into three categories, depending on the type of industry: (a) waste from organic processes (e.g., dairies, chemicals, pharmaceuticals, textiles, foods, and brewing, among others); (b) waste from inorganic processes (chemical and mining industry); and (c) chemical waste (pesticides, herbicides, acids, bases, dyes, fertilizers, pharmaceuticals, among others) [3]. Among the pollutants found in industrial effluents are textile dyes, suspended solids, heavy metals, hydrocarbons, sulfur compounds, chlorinated compounds, fatty acids, surfactants, and phenolic compounds [4]. Phenolic compounds are genotoxic, promote endocrine-disruptive effects, have acute toxicity, and are one of the primary environmental contaminants, especially in water bodies [5]. They can also be released on a smaller scale under natural conditions from decomposing organic matter, but also due to anthropogenic actions by their high use in industry [6]. These compounds are essential in producing chemicals, plastics, oils, pharmaceuticals, dyes, and explosives, among other applications. Consequently, the effluents from these industries exhibit high concentrations of various phenolic compounds [7].
Phenol is the simplest compound in the phenolic family, characterized by a singular aromatic ring and an isolated phenolic hydroxyl group [8]. It presents limited solubility and adsorption to particulate matter. Consequently, the primary modes of transformation for these compounds in the environment involve biodegradation and redox reactions. However, this intricate process can often lead to the formation of compounds that are more environmentally hazardous [9][10]. Common phenol derivatives with high toxicity and low biodegradability such as chlorophenols, aminophenols, bisphenols, and nitrophenols have been detected in waters [11]. The elevated concentrations of phenolic compounds and their toxic derivatives in the environment induce notable alterations in aquatic biota and microbiota, showing significant risks to human health [12].
In many countries, effluent release into the ecosystem needs to be better regulated. Many industrial effluent treatments could be more efficient, and some treatments are costly, which makes correct disposal by small- and medium-sized industries difficult [3][13]. In this sense, several organizations are working to develop sustainable industrial effluent treatment technologies [3], and some techniques have been considered effective in removing pollutants from industrial effluents, such as membrane filtration, adsorption, advanced oxidation processes, and bioremediation [14][15].
Bioremediation is a pollutant cleanup technique focused on biological processes to degrade, reduce, eliminate, modify, detoxify, or transform pollutants into a non-toxic or harmless state [16][17]. Biological treatment with microorganisms has become a preferred method for treating and reducing toxic organic compounds in industrial effluents [18]. Numerous microorganisms have been recognized for their potential in the removal of phenolic compounds from industrial effluents. These include well-established environmental actors, such as bacteria from the genera Pseudomonas and Bacillus, alongside filamentous fungi, such as Aspergillus, with particular attention to white- and brown-rot fungi [19][20][21].
White- and brown-rot fungi, prevalent in nature, are distinguished for their robust lignin-degrading capabilities [22]. These fungi exhibit resilience and proficiency in degrading various recalcitrant compounds, including phenolic compounds. Their significant production of extracellular enzymes such as peroxidases, laccases, and phenol oxidases enables the effective reduction of phenolic compounds into simpler, less ecotoxic molecules [23][24][25]. Studies have underscored the remarkable potential of white- and brown-rot fungi in phenol reduction, achieving the removal of up to 100% of phenolic forms in industrial effluents [26][27].
2. Biochemical Mechanisms Involved in Bioremediation Processes with Filamentous Fungi
Filamentous fungi exhibit numerous mechanisms associated with the bioremediation of phenolic compounds, which may be enzymatic or non-enzymatic. Among the primary non-enzymatic mechanisms, adsorption and biosurfactant production are among the best characterized. Enzymatic mechanisms are linked to fungal metabolism, occurring either intracellularly or extracellularly [28] (Figure 1).
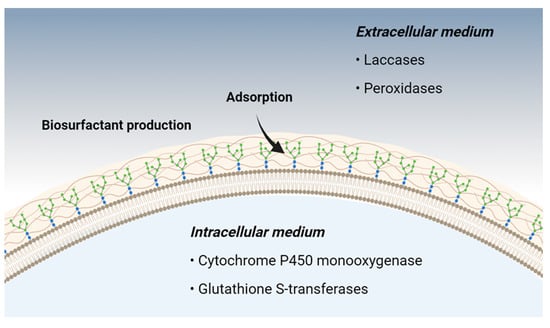
Figure 1. Main metabolic pathways used by filamentous fungi in bioremediation.
Amino-functionalized polysaccharide derivatives present in the fungal cell wall act as adsorbents of toxic phenolic compounds, reducing the bioavailability of these substances in the extracellular environment [29]. Some fungi are capable of producing biosurfactants, substances that reduce the surface tension of liquids, increasing the degradation of poorly soluble and high-molecular-weight compounds, such as petroleum-derived hydrocarbons [30].
Currently, half of the enzymes used in different industrial sectors come from fungal metabolic processes [31]. These enzymes can be of intracellular or extracellular origin and are capable of promoting biotransformation [28]. In this process, several reactions involving hydroxylation, aromatic ring fission, ether cleavage, oxidative coupling products, among others, result in the biotransformation of toxic products into smaller molecules, facilitating bioremediation [32][33].
Intracellular cytochrome P450 enzymes are presented in the form of monooxygenases containing a heme group bound to the cell membrane. These enzymes, in the presence of molecular oxygen and the cofactors NADH or NADPH, are capable of adding an oxygen atom to the substrate. In this process, sequential reactions of molecular oxygen activation, heterolytic cleavage, and formation of a hydroxylated product occur [34]. The ability of cytochrome P450 monooxygenase to catalyze a wide variety of reactions, such as aromatic hydroxyl, dealkylation, epoxidation, and dehalogenation, makes this intracellular enzyme promising in the cleavage of polluting phenolic compounds [35].
Glutathione S-transferase is an intracellular enzyme located in different compartments of the cell, this enzyme is capable of catalyzing the nucleophilic attack of an electrophilic C, N, or S atom in nonpolar compounds using a molecule of glutathione in the reduced form (GSH) [36]. Due to its broad substrate specificity, this enzyme has been studied as an adjuvant in the degradation of xenobiotic compounds [37].
Filamentous fungi, due to their degradative metabolism (heterotrophic), produce extracellular enzymes that are fundamental for the bioconversion of numerous complex substrates [38]. The extracellular enzymes laccases and peroxidases are considered the two most important subclasses of fungal enzymes used in the degradation of xenobiotic compounds, as well as the removal of toxic phenolic substances of industrial origin [39].
Laccases are multi-copper oxidases with broad-spectrum action on substrates capable of degrading phenolic compounds via one-electron oxidation. This enzyme is considered environmentally friendly as it requires molecular oxygen as a co-substrate during catalysis and results in water as the only by-product [40]. Structurally, the enzyme can present a homodimeric, heterodimeric and even multimeric form with a molecular weight (MW) varying between 50 and 110 kDa depending on the microorganism. Fungi produce laccases of MW’s around 70 to 70 kDa with an isoelectric point at pH 4.0, and redox potential of +0.79V [41][42][43]. The high redox power of fungal laccase enables the oxidation of various substrates; for example, phenol has a redox potential ranging between +0.5 and +1.0 V versus a standard hydrogen electrode. In some instances, its oxidation is made possible by transferring electrons to the enzyme’s type 1 (T1) copper [44].
The oxidation reaction of phenolic compounds using a fungal laccase occurs through four copper atoms organized in three active sites (T1, T2, and T3). During enzymatic catalysis, the copper active site in T1 accepts electrons from the substrate, which are then transferred by a cluster composed of the active sites T2 and T3. The electrons in T2 and T3 then reduce O2, leading to the intermediate and transient formation of a peroxide molecule, which soon converts into water, a harmless byproduct of the reaction (Figure 2). The oxidized products can follow different pathways, forming new substrates or even having their toxicity reduced/annulled by the reaction [45]. In addition to phenol, laccases can degrade compounds such as polyphenols, benzenethiols, polyamines, hydroxyindole, and aryldiamines [46][47].
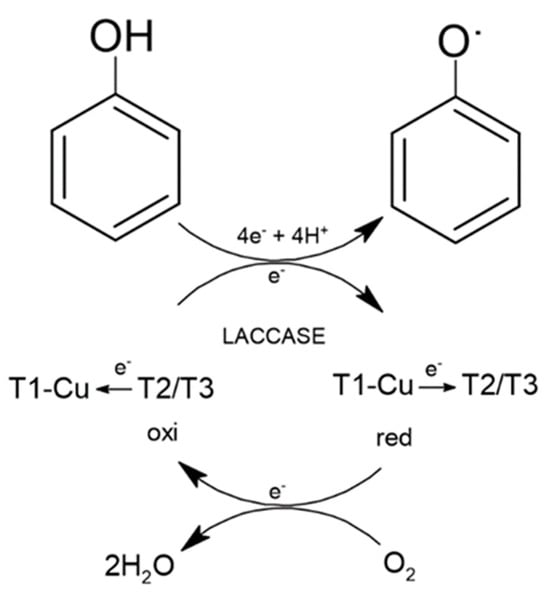
Figure 2. Main chemical reactions of laccase.
Peroxidases and oxidases are extracellular enzymes produced by fungal cells generally in response to the presence of H2O2 and Reactive Oxygen Species (ROS) in periods of oxidative stress. Peroxidase acts by inactivating H2O2 through the transfer of ions and charged radicals, which consequently affects the substrate, resulting in oxidation and hydrolysis [48]. Recent studies have supported the role of glutathione peroxidase and catalase in decomposing plastics, such as polyethylene and polyvinyl chloride, through oxidation reactions [49][50][51].
The main fungal peroxidases are manganese peroxidase (MnP), lignin peroxidase (LiP), versatile peroxidases, and dye-decolorizing peroxidases, with each type depending on the substrate as the reducing agent [52]. Manganese peroxidase is capable of oxidizing aromatic amines and phenolic compounds through the oxidation of an electron and producing more reactive free radicals. This enzyme has been employed in the degradation of recalcitrant aromatic contaminants (industrial dyes), polycyclic aromatic hydrocarbons, chlorophenols, and antibiotics [53].
Lignin peroxidase is capable of cleaving β-O-4’ ether bonds and C-C bonds in lignin, enabling the depolymerization of the molecule, making it susceptible to new biological/chemical actions [54]. The enzyme can also degrade lignin oligomers, including non-phenolic and phenolic compounds [33].
Versatile peroxidase is known for its hybrid activity, combining catalytic functions of MnP and LiP. It oxidizes Mn2+ and non-phenolic compounds with a high redox potential. Additionally, it can oxidize phenolic, non-phenolic, and lignin derivatives without the presence of manganese and without requiring a mediator for oxidation [55][56].
Finally, dye-decolorizing peroxidases (DyPs) are extracellular enzymes that present in their structure a heme group that catalyzes the reduction of hydrogen peroxide in water with the simultaneous oxidation of several other substrates, including anthraquinone dyes and phenolic compounds, as well as non-phenolic compounds [57]. Although the peroxidase family is one of the most studied, little is known about the physiological role and catalytic mechanism of DyPs. Studies have highlighted their importance mainly in the treatment of textile effluents [57][58].
This entry is adapted from the peer-reviewed paper 10.3390/fermentation10030143
References
- Sunkad, S. The Role of Industries in the Development of the Nation. Eur. J. Res. Dev. Sustain. 2021, 2, 55–58.
- Heinz, O.L.; Cunha, M.A.A.; Amorim, J.S.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Barreto-Rodrigues, M. Combined Fungal and Photo-Oxidative Fenton Processes for the Treatment of Wood-Laminate Industrial Waste Effluent. J. Hazard. Mater. 2019, 379, 120790.
- Saravanakumar, K.; De Silva, S.; Santosh, S.S.; Sathiyaseelan, A.; Ganeshalingam, A.; Jamla, M.; Sankaranarayanan, A.; Veeraraghavan, V.P.; MubarakAli, D.; Lee, J.; et al. Impact of Industrial Effluents on the Environment and Human Health and Their Remediation Using MOFs-Based Hybrid Membrane Filtration Techniques. Chemosphere 2022, 307, 135593.
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.G.; Wu, Y. Periphyton Biofilms: A Novel and Natural Biological System for the Effective Removal of Sulphonated Azo Dye Methyl Orange by Synergistic Mechanism. Chemosphere 2017, 167, 236–246.
- Chae, Y.; Kim, L.; Kim, D.; Cui, R.; Lee, J.; An, Y.-J. Deriving Hazardous Concentrations of Phenol in Soil Ecosystems Using a Species Sensitivity Distribution Approach. J. Hazard. Mater. 2020, 399, 123036.
- Mohamad Said, K.A.; Ismail, A.F.; Abdul Karim, Z.; Abdullah, M.S.; Hafeez, A. A Review of Technologies for the Phenolic Compounds Recovery and Phenol Removal from Wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289.
- Gami, A.A.; Shukor, M.Y.; Khalil, K.A.; Dahalan, F.A.; Khalid, A.; Ahmad, S.A. Phenol and Its Toxicity. J. Environ. Microbiol. Toxicol. 2014, 2, 11–23.
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921.
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of Phenol and Cresols to Aquatic Organisms: A Review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456.
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds-Natural Sources, Importance and Applications; InTech: London, UK, 2017.
- Crane, J.L. Distribution and Toxic Potential of Alkylphenols, Nonylphenol Ethoxylates, and Pyrethroids in Minnesota, USA Lake Sediments. Sci. Total Environ. 2021, 776, 145974.
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of Phenolic Compounds from Industrial Waste Water Based on Membrane-Based Technologies. J. Ind. Eng. Chem. 2019, 71, 1–18.
- Nidheesh, P.V.; Ravindran, V.; Gopinath, A.; Kumar, M.S. Emerging Technologies for Mixed Industrial Wastewater Treatment in Developing Countries: An Overview. Environ. Qual. Manag. 2022, 31, 121–141.
- Ken, D.S.; Sinha, A. Dimensionally Stable Anode (Ti/RuO2) Mediated Electro-Oxidation and Multi-Response Optimization Study for Remediation of Coke-Oven Wastewater. J. Environ. Chem. Eng. 2021, 9, 105025.
- Chalaris, M.; Gkika, D.A.; Tolkou, A.K.; Kyzas, G.Z. Advancements and Sustainable Strategies for the Treatment and Management of Wastewaters from Metallurgical Industries: An Overview. Environ. Sci. Pollut. Res. 2023, 30, 119627–119653.
- Mora-Ravelo, S.G. Bioremediation of Wastewater for Reutilization in Agricultural Systems: A Review. Appl. Ecol. Environ. Res. 2017, 15, 33–50.
- Tripathi, S.; Sharma, P.; Purchase, D.; Chandra, R. Distillery Wastewater Detoxification and Management through Phytoremediation Employing Ricinus communis L. Bioresour. Technol. 2021, 333, 125192.
- Kumar, V.; Thakur, I.S.; Shah, M.P. Bioremediation Approaches for Treatment of Pulp and Paper Industry Wastewater: Recent Advances and Challenges. In Microbial Bioremediation & Biodegradation; Springer: Singapore, 2020; pp. 1–48.
- Dong, R.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Removal of Phenol from Aqueous Solution Using Acid-Modified Pseudomonas putida-sepiolite/ZIF-8 Bio-Nanocomposites. Chemosphere 2020, 239, 124708.
- Kubisch, C.; Ochsenreither, K. Detoxification of a Pyrolytic Aqueous Condensate from Wheat Straw for Utilization as Substrate in Aspergillus oryzae DSM 1863 Cultivations. Biotechnol. Biofuels Bioprod. 2022, 15, 18.
- Swain, G.; Sonwani, R.K.; Singh, R.S.; Jaiswal, R.P.; Rai, B.N. Removal of 4-Chlorophenol by Bacillus flexus as Free and Immobilized System: Effect of Process Variables and Kinetic Study. Environ. Technol. Innov. 2021, 21, 101356.
- Castaño, J.D.; Muñoz-Muñoz, N.; Kim, Y.M.; Liu, J.; Yang, L.; Schilling, J.S. Metabolomics Highlights Different Life History Strategies of White and Brown Rot Wood-Degrading Fungi. mSphere 2022, 7, e00545-22.
- Grelska, A.; Noszczyńska, M. White Rot Fungi Can Be a Promising Tool for Removal of Bisphenol A, Bisphenol S, and Nonylphenol from Wastewater. Environ. Sci. Pollut. Res. 2020, 27, 39958–39976.
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; Polizeli, M.d.L.T.d.M.; Bracht, A.; et al. A Highly Reusable MANAE-Agarose-Immobilized Pleurotus ostreatus Laccase for Degradation of Bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351.
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The Effect of Operational Parameters on the Biodegradation of Bisphenols by Trametes versicolor Laccase Immobilized on Hippospongia communis Spongin Scaffolds. Sci. Total Environ. 2018, 615, 784–795.
- Latif, W.; Ciniglia, C.; Iovinella, M.; Shafiq, M.; Papa, S. Role of White Rot Fungi in Industrial Wastewater Treatment: A Review. Appl. Sci. 2023, 13, 8318.
- Nurika, I.; Suhartini, S.; Barker, G.C. Biotransformation of Tropical Lignocellulosic Feedstock Using the Brown Rot Fungus Serpula lacrymans. Waste Biomass Valorization 2020, 11, 2689–2700.
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous Fungi for Sustainable Remediation of Pharmaceutical Compounds, Heavy Metal and Oil Hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973.
- Legorreta-Castañeda, A.; Lucho-Constantino, C.; Beltrán-Hernández, R.; Coronel-Olivares, C.; Vázquez-Rodríguez, G. Biosorption of Water Pollutants by Fungal Pellets. Water 2020, 12, 1155.
- Zainab, R.; Hasnain, M.; Ali, F.; Dias, D.A.; El-Keblawy, A.; Abideen, Z. Exploring the Bioremediation Capability of Petroleum-Contaminated Soils for Enhanced Environmental Sustainability and Minimization of Ecotoxicological Concerns. Environ. Sci. Pollut. Res. 2023, 30, 104933–104957.
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2021, 8, 23.
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Recent Advances in Fungal Xenobiotic Metabolism: Enzymes and Applications. World J. Microbiol. Biotechnol. 2023, 39, 296.
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Meyer, A.S.; Raj, A. Bioremediation of Lignin Derivatives and Phenolics in Wastewater with Lignin Modifying Enzymes: Status, Opportunities and Challenges. Sci. Total Environ. 2021, 777, 145988.
- Urlacher, V.B.; Girhard, M. Cytochrome P450 Monooxygenases in Biotechnology and Synthetic Biology. Trends Biotechnol. 2019, 37, 882–897.
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of Organic Pollutants by White Rot Fungal Cytochrome P450: The Role and Mechanism of CYP450 in Biodegradation. Chemosphere 2022, 301, 134776.
- Raza, H. Dual Localization of Glutathione S-transferase in the Cytosol and Mitochondria: Implications in Oxidative Stress, Toxicity and Disease. FEBS J. 2011, 278, 4243–4251.
- Koirala, B.K.S.; Moural, T.; Zhu, F. Functional and Structural Diversity of Insect Glutathione S-Transferases in Xenobiotic Adaptation. Int. J. Biol. Sci. 2022, 18, 5713–5723.
- Ferreira, J.A.; Varjani, S.; Taherzadeh, M.J. A Critical Review on the Ubiquitous Role of Filamentous Fungi in Pollution Mitigation. Curr. Pollut. Rep. 2020, 6, 295–309.
- Verma, M.L.; Thakur, M.; Randhawa, J.S.; Sharma, D.; Thakur, A.; Meehnian, H.; Jana, A.K. Biotechnological Applications of Fungal Enzymes with Special Reference to Bioremediation. Environ. Biotechnol. 2020, 2, 221–247.
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in Food Industry: Bioprocessing, Potential Industrial and Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 8, 222.
- Haugland, J.O.; Kinney, K.A.; Johnson, W.H.; Camino, M.M.A.; Whitman, C.P.; Lawler, D.F. Laccase Removal of 2-chlorophenol and Sulfamethoxazole in Municipal Wastewater. Water Environ. Res. 2019, 91, 281–291.
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; RodrÍguez-Vázquez, R.; Delgado-Boada, J.M. Fungal Laccases. Fungal Biol. Rev. 2013, 27, 67–82.
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, Function, and Potential Application in Water Bioremediation. Microb. Cell Fact. 2019, 18, 200.
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A Never-Ending Story. Cell. Mol. Life Sci. 2010, 67, 369–385.
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with Laccases: An Updated Overview. Catalysts 2020, 11, 26.
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705.
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various Types and Applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672.
- Okal, E.J.; Heng, G.; Magige, E.A.; Khan, S.; Wu, S.; Ge, Z.; Zhang, T.; Mortimer, P.E.; Xu, J. Insights into the Mechanisms Involved in the Fungal Degradation of Plastics. Ecotoxicol. Environ. Saf. 2023, 262, 115202.
- Zeghal, E.; Vaksmaa, A.; Vielfaure, H.; Boekhout, T.; Niemann, H. The Potential Role of Marine Fungi in Plastic Degradation—A Review. Front. Mar. Sci. 2021, 8, 738877.
- Gao, R.; Pan, H.; Lian, J. Recent Advances in the Discovery, Characterization, and Engineering of Poly(Ethylene Terephthalate) (PET) Hydrolases. Enzym. Microb. Technol. 2021, 150, 109868.
- Zhang, Z.; Peng, H.; Yang, D.; Zhang, G.; Zhang, J.; Ju, F. Polyvinyl Chloride Degradation by a Bacterium Isolated from the Gut of Insect Larvae. Nat. Commun. 2022, 13, 5360.
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180.
- Kumar, A.; Arora, P.K. Biotechnological Applications of Manganese Peroxidases for Sustainable Management. Front. Environ. Sci. 2022, 10, 875157.
- Pham, L.T.M.; Deng, K.; Northen, T.R.; Singer, S.W.; Adams, P.D.; Simmons, B.A.; Sale, K.L. Experimental and Theoretical Insights into the Effects of PH on Catalysis of Bond-Cleavage by the Lignin Peroxidase Isozyme H8 from Phanerochaete chrysosporium. Biotechnol. Biofuels 2021, 14, 108.
- Schneider, W.D.H.; Camassola, M.; Fontana, R.C. How Ligninolytic Enzymes Can Help in the Degradation of Biomass Polysaccharides, Cleavage, and Catalytic Mechanisms? In Polysaccharide-Degrading Biocatalysts; Elsevier: Amsterdam, The Netherlands, 2023; pp. 177–190.
- Knop, D.; Levinson, D.; Makovitzki, A.; Agami, A.; Lerer, E.; Mimran, A.; Yarden, O.; Hadar, Y. Limits of Versatility of Versatile Peroxidase. Appl. Environ. Microbiol. 2016, 82, 4070–4080.
- Silva, D.; Rodrigues, C.F.; Lorena, C.; Borges, P.T.; Martins, L.O. Biocatalysis for Biorefineries: The Case of Dye-Decolorizing Peroxidases. Biotechnol. Adv. 2023, 65, 108153.
- Kita, D.M.; Giovanella, P.; Yoshinaga, T.T.; Pellizzer, E.P.; Sette, L.D. Antarctic Fungi Applied to Textile Dye Bioremediation. An. Acad. Bras. Cienc. 2022, 94, e20210234.
This entry is offline, you can click here to edit this entry!
