Phase change materials (PCM) were known already in the 1970s (similarly to geopolymers), but their development is observed today. This is related to the possibility of obtaining a stable phase as well as the possibility of optimization of construction materials concerning the construction needs. These materials can change their physical state depending on the ambient temperature. The most common method is liquid-solid conversion. In the area of application of phase change materials in the construction industry, examples of such materials include organic materials (paraffin, fatty acids), inorganic materials, and eutectics.
1. Introduction
Energy is a key element in assessing the progress of society around the world in various aspects such as technological development, environmental protection, and economic progress. The continuous depletion of non-renewable energy resources leads to the major problem of global warming. This is prompting scientists to change their approach for better and cleaner use of energy. With a higher standard of living and technological growth, the electricity demand is increasing day by day, leading to excessive consumption of fossil fuels. Minimizing the amount of consumed energy can reduce environmental pollution and also have a positive impact on the energy market.
The production of the most important building material of the 20th century, Portland cement, is associated with significant environmental pollution and energy problems. It is produced at very high temperatures of the order of 1400–1500 °C. During production, significant amounts of carbon dioxide and highly toxic nitrogen oxides are emitted into the atmosphere. The building sector is responsible for huge energy consumption which is estimated to be around 40% worldwide—of which, residential buildings consume 27% of energy and contribute 17% of CO
2 emissions to the environment [
1]. This is due to rapid population growth and changing standards of lifestyle. Such high energy demand is reflected in significant environmental pollution and degradation. To avoid the negative effects of excessive energy consumption, sustainable building materials are increasingly being developed [
2]. In recent years, sustainable technologies and materials for the construction sector have begun to develop very quickly in Poland and in the rest of the world.
In recent years, alkali-activated binders/geopolymer cement and concrete seem to be an alternative to conventional concrete. Alkali activated binders are produced by alkaline activation of aluminosilicate raw materials such as blast furnace slag (GGBFS), fly ash (FA), metakaolin (MK), etc. These binders can solve the problems of the construction industry and waste generation. Therefore, since calcium-rich raw materials, especially slags, are limited, more attention is paid to raw materials with a low calcium content, from which geopolymers are produced [
3]. In 1978, Joseph Davidovitz first coined the term “geopolymer” [
4]. The term geopolymer covers the class of modern, environmentally-friendly, inorganic, amorphous, synthetic aluminosilicates with specific composition and properties [
5]. Geopolymers are three-dimensional aluminosilicate framework structures from amorphous to semi-crystalline, which are formed by a combination of [SiO
4]
4− and [AlO
4]
5− tetrahedra. The structure remains electrically neutral as a result of the replacement of aluminum by silicon in the tetrahedral layer with available alkalis, such as Na
+ [
6]. The mechanism of setting and hardening of geopolymer is not fully understood. The geopolymerization process can be carried out by dissolving raw materials containing aluminosilicate in alkaline solutions, which leads to the formation of aluminate and silicate monomers. Then they turn into oligomers and then into geopolymers [
5]. Research on geopolymers is conducted mainly to replace conventional cement with them and, consequently, to use them on a large scale in construction. Concrete made from geopolymer cements has received considerable attention because of their low cost, good engineering properties as well as low energy consumption. Materials of this type are used in specific areas, where their unique properties, such as very high fire resistance, chemical corrosion resistance, high mechanical strength, and excellent durability, are important [
3,
7,
8,
9,
10,
11,
12]. Geopolymers are characterized by better performance compared to concrete and, importantly, large amounts of by-products (fly ash, coal ash, and blast furnace slag) are used on production [
13,
14,
15,
16,
17,
18].
Despite the existence of building materials that are more environmentally friendly than conventional cement, there is still a need to improve the energy efficiency of buildings and reduce emissions of substances that are harmful to the earth’s atmosphere.
One of the solutions to reduce the negative impact on the environment is the application of phase change materials (PCM) [
19]. Due to the declining supply of fossil fuels, in the last few years, phase change materials have attracted the interest of a wide range of researchers, organizations, and benefactors as they store thermal energy and release it when needed [
20,
21]. This is evidenced by research in various fields, such as the electronics industry [
22], photovoltaic and solar systems [
23,
24], hot water systems [
25].
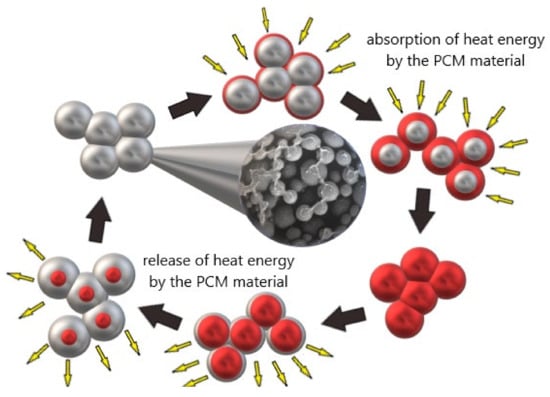
Phase change materials (PCM) were known already in the 1970s (similarly to geopolymers), but their development is observed today. This is related to the possibility of obtaining a stable phase as well as the possibility of optimization of construction materials concerning the construction needs. These materials can change their physical state depending on the ambient temperature. The most common method is liquid-solid conversion. In the area of application of phase change materials in the construction industry, examples of such materials include organic materials (paraffin, fatty acids), inorganic materials, and eutectics. Most inorganic PCMs are characterized by large volume changes and potential subcooling, which has led to the wider use of organic PCMs in combination with concrete [
26]. The most commonly used organic materials are in the form of microcapsules. Organic paraffin was considered as the best phase change material for concrete mortar due to its stability and inertness in an alkaline environment [
27]. However, this material also has some limitations, such as low thermal conductivity, flammability, incompatibility with plastics, and large volume changes [
28]. The choice of PCM material and the method of its incorporation is determined by e.g., the temperature range of phase transitions, the effective heat capacity, and the stability of the material during cyclic transitions. Phase change materials can have up to several times higher heat capacity than traditional construction materials. It is extremely important to use phase change materials in appropriate types of building materials. As research indicates, geopolymers may be a better option for these types of systems than traditional building materials [
29]. The way PCM works in geopolymers is shown schematically in
Figure 1.
Figure 1. The mode of action of phase change materials.
The incorporation of microencapsulated phase change materials (MPCMs) in structural applications can improve the thermal energy storage capacity, thereby lowering the energy demand in buildings [
30]. The addition of MPCM reduces the workability and mechanical strength of concrete. However, the type, quantity, and proportion of raw materials curing time, and temperature should be considered when formulating geopolymer concrete (GPC) [
31].
Phase change materials have a high latent heat storage density, so they can absorb thermal energy when they transform from a solid to a liquid or release it when they transform to a solid state. Due to this property, PCM can function as a heating and cooling system in the building industry. During the day it absorbs excess thermal energy in a building component through a melting process, while at reduced temperatures (at night) it solidifies and releases thermal energy back into the environment.
PCM can be introduced into building components in three ways. The first method is to add it directly during the mixing process. Another method is to immerse the building structure in liquid PCM. The third method is the most advanced and popular, and consists of encapsulation of micro or macro PCM. It allows for better particle dispersion, reduces external volume changes, but also eliminates interaction between the phase change material and the starting material. Microencapsulation of phase change material is now an industrialized process, so it is very expensive and the product itself has been limited to only a few companies worldwide [
32]. The addition of phase change materials to the inner layer of the partition will produce several beneficial phenomena not only related to thermal comfort inside the building [
33,
34,
35] but also, among others, that the addition in the form of PCM reduces thermal gradients and unifies the temperature inside the concrete mix, which may reduce the risk of cracking. Phase change materials can also be added to interior finishes such as gypsum wallboard [
36,
37]. The addition of such materials results in a reduction of costs of heating or cooling buildings, and an increase in energy efficiency in rooms as well as increased comfort for users.
2. Characteristics and Classification of Phase Change Materials
A material with a phase change is a compound or a group of compounds capable of absorbing and releasing large amounts of energy depending on the phase change they are currently in. The basic properties characterizing PCM are primarily the ability to heat accumulation and thermal conductivity of the substance. In addition, the behavior of the substance in superheated and subcooled conditions. These materials are characterized by a certain range and value of temperature at which the phase transformation occurs [
38,
39]. Phase change materials are also known as materials with the ability to accumulate so-called “latent” heat. The transfer of thermal energy takes place when a material changes from one phase to another, i.e., from a liquid to a solid or from a solid to a liquid state. Initially, these materials behave like conventional heat accumulators such as water—they increase in temperature as heat is supplied. However, PCMs absorb and release heat at an almost constant temperature. They can absorb 5 to 14 times more heat than standard materials [
40]. To realize the full potential of phase change materials, they must meet some requirements from thermal, physical, chemical, and kinetic aspects. In addition, an important aspect of using a particular type of material is the economic aspect and the availability of materials [
41,
42].
Considering thermal issues, these materials should have high latent heat per unit volume. This aspect is extremely important because of the potential size of the heat accumulator. With high latent heat, the size of the heat storage can be minimized. The high thermal conductivity of such a material significantly facilitates the heat loading and unloading process. Small volume changes during phase transitions and low vapor pressure at working temperatures are other basic characteristics that PCMs should meet from the physical point of view. The basic chemical requirements for PCM are non-toxicity, non-flammability, and low probability of an explosion. During selections of phase change materials, it must be remembered not to subcool them, as this significantly impedes the dissipation of stored heat. Subcooling of the PCM by as little as 5–10 °C can cause complete blocking of the ability to transfer stored heat [
40,
41,
42,
43].
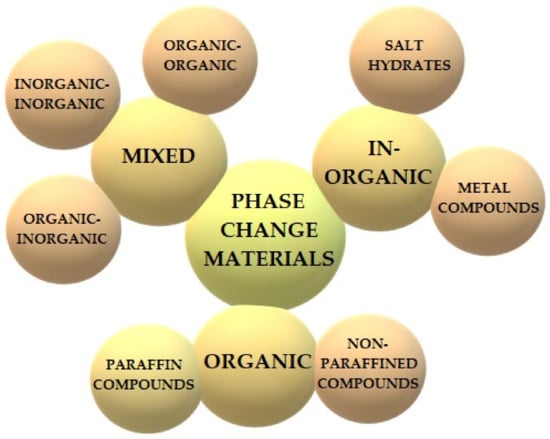
The basic criterion for the distinction of phase change materials is the structure and chemical composition. There are three basic classifications of phase change materials: organic, non-organic materials, and mixed (eutectic). Figure 2 shows the main types of phase change materials.
Figure 2. Classifications of phase change materials.
The use of a particular type of PCM has its advantages and disadvantages compared to other types. Based on the literature sources, several advantages and disadvantages for organic and inorganic PCMs were summarized. Starting from organic PCMs, their greatest advantages are compatibility with conventional common materials and high synthesis temperature [
24]. In addition, they have self-nucleating properties and are safe and non-toxic. The disadvantages of organic PCMs include flammability and relatively low volumetric capacity to store latent heat [
44]. They are also characterized by low thermal conductivity. In the case of non-organic materials, their greatest advantage is their low cost and high availability. Unlike organic materials, they are characterized by high latent heat storage capacity and high thermal conductivity. Like organic materials, they have a high synthesis temperature. Considering the most important disadvantages of inorganic phase change materials are their corrosion and frequent volume change in some mixtures. In addition, in the case of this type of PCM there is a high risk of subcooling of the substance [
21].
Currently, dozens of large companies are involved in the production of phase change materials as well as finished products. The most well-known include, among others: Rubitherm, Doerken, BASF (Berlin, Germany), PCM Products (Peterborough, UK), PureTemp LLC (Minneapolis, USA), Climator (Skovde, Sweden), Cristopia (Vence, France), Mitsubishi Chemical (Kurashiki, Japan), TEAP Energy (Melbourne, Australia), PCM (Suzhou, China), PlusPolymer (Haryna, India) [
45].
A very interesting solution is the PCM materials introduced by e.g., RUBITHERM in the form of dry powder (PX) and granulates (GR) in an inorganic matrix. These should not be confused with microencapsulated PCM. Their appearance is shown in
Figure 3. The organic part in PX material is about 60% while in GR variety it is 30%. The particle size of the PX inorganic carrier matrix is 200 µm. According to the manufacturer, PX materials are also more economical compared to microencapsulated PCM, but it should be taken into account that microencapsulated PCM cannot be replaced. The diameter of GR granules is about 1–3 mm. GR is used in the food and beverage industry and as a filler for accumulation plates [
46].
Figure 3. PCM granules manufactured by RUBITHERM [
46].
Various manufacturers also offer PCM materials in other forms: encapsulated in plastic beads, stainless steel, steel tubes, pouches, ceramic materials. There are more and more solutions on the market, but many of them are designed and manufactured for food manufacturers and packaging manufacturers. There is also great development in the field of energy storage. However, there is still a lot of room for improvement when it comes to building materials. It should be remembered that this is a huge sector that can contribute to a significant increase in demand for MSM and consequently reduce the environmental impact on people. But this requires continuous improvement of these materials and adapting them to changing technologies.
3. Methods of Incorporation of PCM into Geopolymers and Concrete
One of the most significant problems in creating geopolymer composites with PCMs is how to place the PCM materials in the matrix. This is also true for conventional concrete composites. Using the wrong form may result in the little effect of the PCM addition, or the entire PCM material may be destroyed or removed after some time.
There are 4 main methods of incorporation of PCM into geopolymer:
-
Microencapsulation (PCMs are enclosed in spherical shells),
-
Shape stabilized PCMs (Molten PCM is directly absorbed into powdered porous structure like graphite powder, silica fume, etc. having higher thermal conductivity),
-
Porous aggregate inclusions (Molten PCM is absorbed or impregnated into porous light weight aggregates having large surface area and aggregate size such as expanded perlite, expanded clay, pumice, etc.),
-
Macroencapsulation (PCMs are incorporated into building components such as roofs or walls).
The use of stabilized forms of PCM (i.e., encapsulated or impregnated PCM) in porous support materials, such as expanded graphite or expanded perlite, in geopolymer mortar, can result in better mechanical and thermal properties compared to geopolymer containing neat PCM alone [
2]. Besides, usage of PCM in free form—not encapsulated or encapsulated—can lead to several problems related to the spillage of PCM content outside the materials.
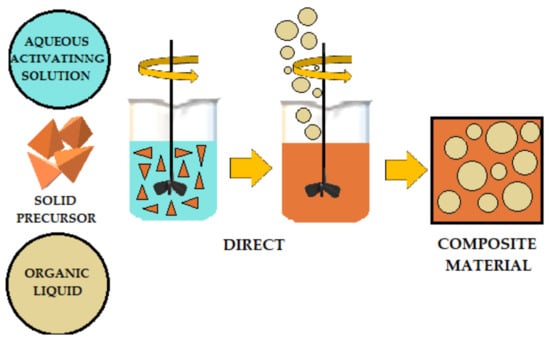
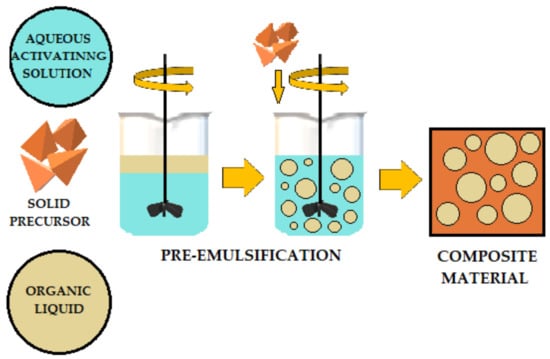
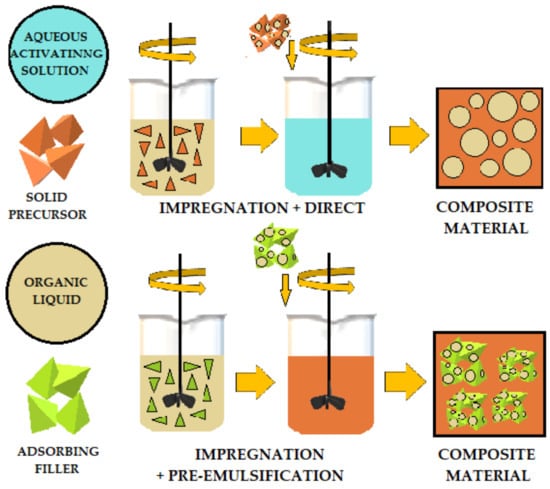
The literature describes various methods for adding organic liquids to cement-based or geopolymer-based materials.
Figure 4,
Figure 5 and
Figure 6 schematically illustrates three ways to introduce organic liquids into ordinary Portland cement (OPC) or geopolymers (GP).
Table 1 compares these methods and describes their advantages and disadvantages [
49].
Figure 4. Schematic of direct addition of organic liquids to OPC (ordinary Portland cement) and AAM (alkali-activated) materials.
Figure 5. Preemulsification schematic of organic liquids added to OPC (ordinary Portland cement) and AAM (alkali-activated) materials.
Figure 6. Combined method of adding organic liquids added to OPC (ordinary Portland cement) and AAM (alkali activated) materials.
Table 1. Advantages and disadvantages of three incorporation processing routes (based on [
34,
49]).
| |
Advantages
|
Disadvantages
|
|
Direct method
|
-
No additives required,
-
Easy, one-step process,
-
A large amount of oil
|
|
|
Preemulsification
|
|
|
|
Impregnation
|
|
|
4. PCM in Geopolymers—Effect on Insulation Properties
The use of PCM materials in geopolymers were widely described in [
50,
51,
52], among others. This idea came about the same time that PCMs were added and studied in conventional concretes. Since it is, however, a different type of matrix, often with a very high pH, the issue of PCM addition to geopolymers should be considered separately from concretes based on Portland cement, even though the properties of such composites and their application may be similar.
Selected results of studies on geopolymers with PCM are presented below, mainly with respect to the influence of PCM on thermal properties of geopolymer composites. Only selected results are presented, and the focus is on presenting different forms in which PCM were introduced e.g., powder, encapsulated in graphite, encapsulated in polyurethane foams, microcapsules, encapsulated PCM, etc. Table 2 summarizes the most important information.
Table 2. Research (selected) summary of geopolymer containing PCM.
|
Name of PCM
|
Producer
|
Core Material of PCM
|
Melting Point of PCM [°C]
|
Heat Capacity of PCM [kJ/kg]
|
Form of Applied Matrix for PCM before Incorporation into Geopolymer
|
Application Effect in Material
|
References
|
|
RT31
|
Rubiterm, Berlin, Germany
|
Paraffin
|
31
|
165
|
Polyurethane foam, covered with geopolymer paste
|
Protection against overheating as a passive cooling system
|
[53]
|
|
MPCM 28D
|
Microtek Laboratories, Inc., Dayton, OH, USA
|
Paraffin
|
28
|
180–195
|
Polymer shells (microcapsules)
|
Damping function, less temperature fluctuation; increase in heat capacity
|
[54]
|
|
PCM
|
Avantis Company La- Boratorium Berhad, Ipoh, Perak, Malaysia
|
Paraffin
|
No data
|
189
|
Expanded graphite + CaCl2/sodium silicate coating
(microcapsules)
|
Storing thermal energy and releasing it as latent heat
|
[55]
|
|
PCM
|
No data
|
Eutectic BaCl2 + KCl + NaCl
|
No data
|
215
|
ZYP coating or Aremco coating or ZAG coatin + geopolymer shells
|
Reduction in latent heat of all samples
|
[56]
|
|
PCM
|
No data
|
Paraffin
|
25
|
230
|
Commercial synthetic rubber emulsion (Sika Latex), commercial liquid waterproofing membrane (Weber dry-lastick), polyester resin adhesive with hardener and catalyst
|
The aggregate covered with PCM material allows for the storage of thermal energy and return it as latent hea
|
[19]
|
|
PS-DVB/RT27
|
Rubitherm, Berlin, Germany
|
Paraffin
|
24.9
|
100
|
Polymer shells (microcapsules)
|
Reduction in energy consumption for internal temperature stabilisation by 18.5 ± 0.3%
|
[60]
|
|
Micronal DS-5038X
|
Microtek Laboratories, Inc. Dayton, OH, USA
|
Paraffin
|
24.7
|
110
|
Polymer shells (microcapsules)
|
Reduction in energy consumption for internal temperature stabilisation by 20.1 ± 0.7%
|
[60]
|
|
RT27
|
Rubitherm, Berlin, Germany
|
Paraffin
|
24.9
|
100
|
Composite made by vacuum mixing hydrophobic expanded perlite (EP) and paraffin in a 1:1 ratio
|
Geopolymer foam concrete (GFC) showed little temperature variation and little loss
|
[30]
|
This entry is adapted from the peer-reviewed paper 10.3390/ma14206044