Marine biofouling is a major concern for the maritime industry, environment, and human health. Considering that biocides currently used in marine coatings to prevent this phenomenon are toxic to the marine environment, the search for antifoulants with environmentally safe properties is needed. Some natural and synthetic flavonoids have been evaluated over the last few years for their potential to prevent the settlement and/or the growth of marine organisms on submerged structures, thereby preventing marine biofouling.
- flavonoids
- natural
- synthesis
- biofouling
- antifouling activity
1. Introduction
Marine biofouling is a consequence of the settlement and accumulation of adhesive marine organisms’ forms on submerged surfaces, namely on ships, causing huge concerns for the maritime industry, the marine environment, and human health [1][2]. This phenomenon causes huge economic impacts for maritime industries, since the increase in a ship’s roughness leads to an increase in fuel consumption and consequently to extra fuel costs. Moreover, the damage to ships due to discoloration and corrosion are also notorious, and a longer drydock period is necessary to repair the ship [3]. Marine biofouling is also responsible for environmental and health concerns, as it promotes an increase in the emission of polluting gases and the spread of invasive species to other geographical locations, threatening the native species, reducing the biodiversity, and causing the introduction of diseases [3][4][5]. Considering the huge impact of marine biofouling, some antifouling strategies have been adopted to prevent this phenomenon. Since the 1960s, the incorporation of tributyltin (TBT) in marine coatings were used by the maritime industries worldwide, as TBT was able to control a broad spectrum of fouling organisms. However, TBT has been demonstrated to cause impairments in the growth, development, reproduction, and survival of many marine species, and therefore, the use of this biocide was completely banned by the International Maritime Organization in 2008 [6]. As an alternative to the use of TBT and other organotins, some old-fashioned techniques, namely using copper and zinc, and booster biocides, including Irgarol 1051, Zinc pyrithione, Chlorothalonil, and Sea Nine 211, have been used in marine coatings. However, although some of these molecules were described as environmentally friendly biocides, several toxic effects have been attributed to these alternative biocides [7].
Since the antifouling compounds used have proven to be harmful to the marine environment, the search for new more sustainable alternatives is urgent. One of these strategies can be the use of natural and nature-inspired compounds, namely flavonoids, which have shown interesting antifouling activity when tested against some biofouling marine organisms [8]. Flavonoids are natural polyphenols divided into several subclasses, including chalcones, flavonols, flavones, flavanones, isoflavones, flavans, flavanols, and flavanonols (Figure 1), which are not only commonly found in terrestrial plants, but also found in marine sources [9]. These compounds have been widely described over the last decades for having a wide range of biological properties, namely anticancer, antimicrobial, anti-inflammatory, antidiabetic, antioxidant, cardioprotective, and neuroprotective [9][10][11][12]. Despite the wide range of biological activities attributed to flavonoids over the last few decades, few studies reported their potential as antifoulants. The first paper reporting an antifouling assay using a flavonoid was carried out in 1989 [13]. Until now, 21 research papers described the antifouling potential of flavonoids obtained from marine and terrestrial sources, as well as synthetic ones, through the inhibition of the settlement of macrofouling species, namely mussels, barnacles, and algae, as well as their activity against microfouling organisms, including marine bacteria and diatoms.

Figure 1. Examples of subclasses of flavonoids.
2. Antifouling Flavonoids
A total of 106 flavonoids with antifouling activity against macrofouling (mussels, barnacles, algae) and microfouling (diatoms, bacteria, protozoans) species have been reported, including mainly chalcones, flavonols, and flavones. Other classes of flavonoids with antifouling potential were also reported but less represented, namely flavanone, isoflavone, flavan, flavanol, and flavanonol derivatives (Figure 2A). Most of the studies (60%) were performed using microfouling species (namely marine bacteria and diatoms), and only 24% of the compounds were evaluated against macrofouling organisms, mainly mussel and barnacle species. Nonetheless, 16% of the tested compounds were evaluated against both macrofouling and microfouling organisms (Figure 2B). As shown in Figure 2C, most of the compounds were obtained through synthesis in a laboratory (55%), followed by natural flavonoids (26%) and commercial compounds (19%). Among natural flavonoids, the percentage of antifouling flavonoids obtained from marine sources is almost as high as those from terrestrial sources, as shown in Figure 2D.
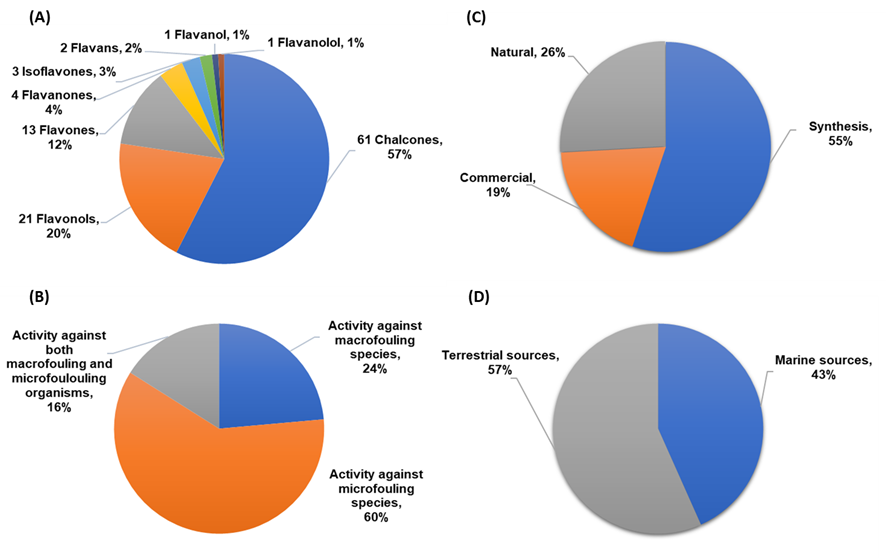
Figure 2. (A) Classes of flavonoids with antifouling activity. (B) Bioactivity evaluated for antifouling flavonoids against macrofouler, microfouler, or both types of fouling organisms. (C) Basis of flavonoids studied for antifouling activity. (D) Source of natural flavonoids.
2.1. Chalcones
A total of 61 chalcones (1-61) with antifouling potential have been reported [14][15][16][17][18]. Although this class of flavonoids is common in nature, all chalcones reported to have antifouling activity were obtained by chemical synthesis. The structures of chalcones with antifouling potential are shown in Figure 3.
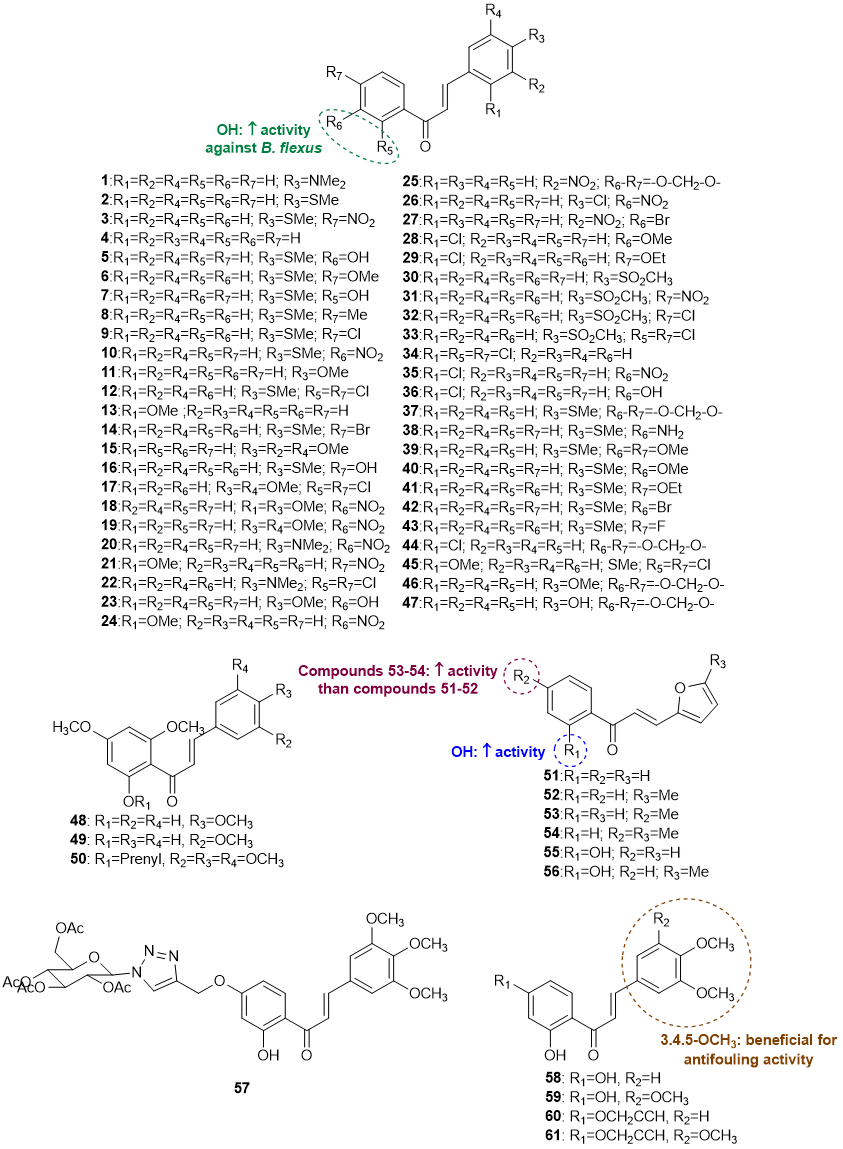
Figure 3. Structures of chalcones 1-61 with antifouling activity and SAR considerations.
2.2. Flavonols
Flavonols comprise the second most reported class of flavonoids with an effect in the prevention of marine biofouling. A total of 21 flavonols (62-81) with antifouling activity [13][19][20][21][22][23][24]. The structures of flavonols with antifouling potential are presented in Figure 4.
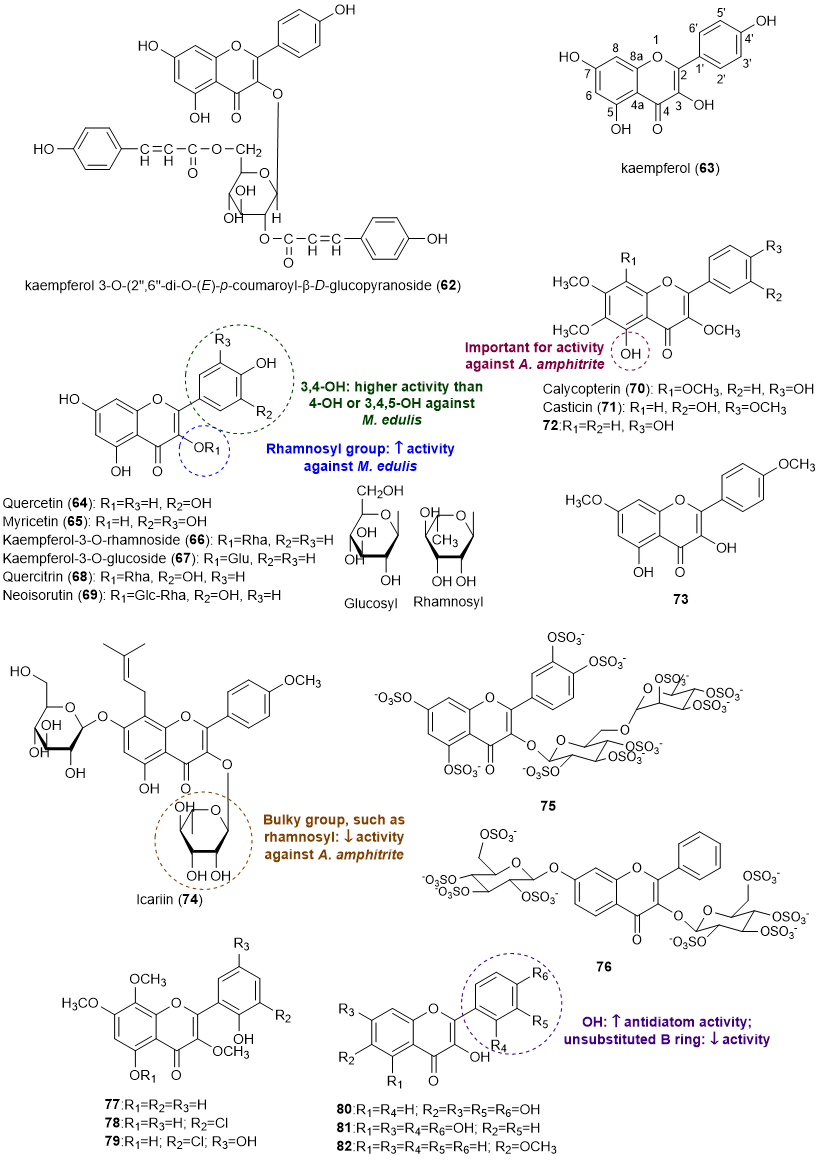
Figure 4. Structures of flavonols 62-82 with antifouling activity and some SAR considerations. Compounds 63, 64 and 77-79 were isolated from marine sources.
2.3. Flavones
Thirteen flavones (83-95) were also found to exert some influence in the prevention of marine biofouling [18][19][20][24][25][26][27][28], being the structures of compounds presented in Figure 5.
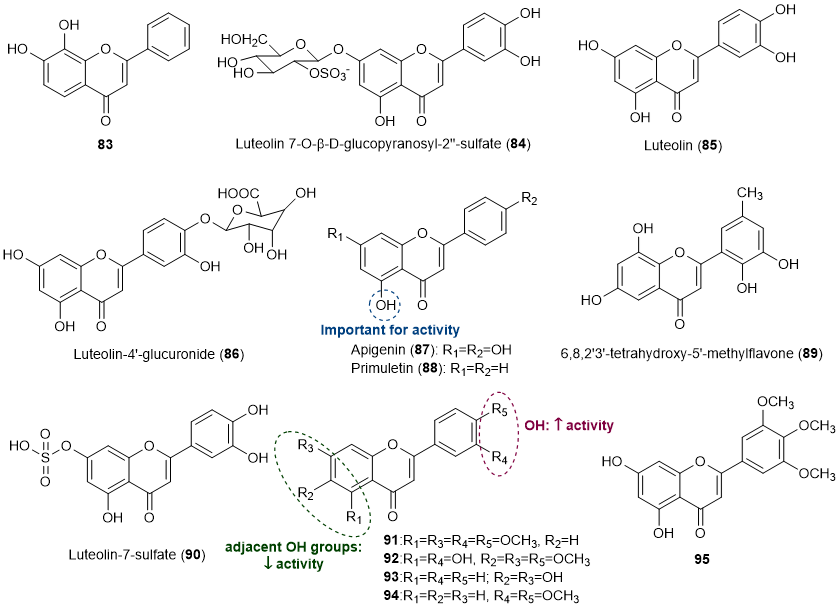
Figure 5. Structures of flavones 83-95 with antifouling activity and some SAR considerations. Compounds 84-86, 89 and 90 were isolated from marine sources.
2.4. Flavanones
Only four flavanones (96-99) with antifouling activity have been reported so far (Figure 6) [19][29][30].
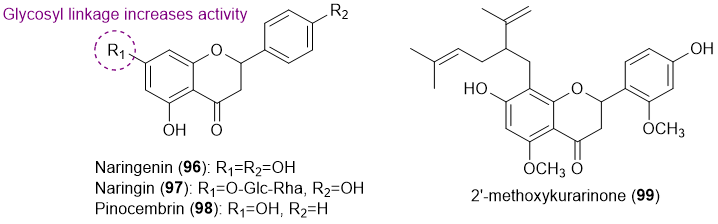
Figure 6. Structures of flavanones 96-99 with antifouling activity.
2.5. Isoflavones
A total of three isoflavones (100-102) with antifouling activity were described (Figure 7) [19][20][21][24][29].
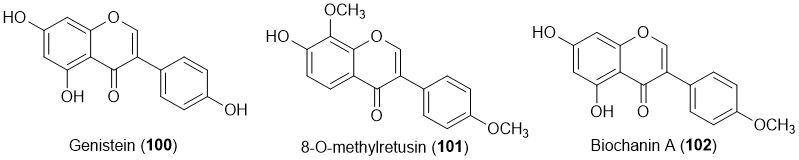
Figure 7. Structures of isoflavones 100-102 with antifouling activity. Isoflavone 101 was isolated from marine sources.
2.6. Other Flavonoids
Other flavonoids, including two flavans (103-104) [31][32], one flavanol (105) [21], and one flavanonol (106) [33] (Figure 8), also showed some potential in marine biofouling prevention.
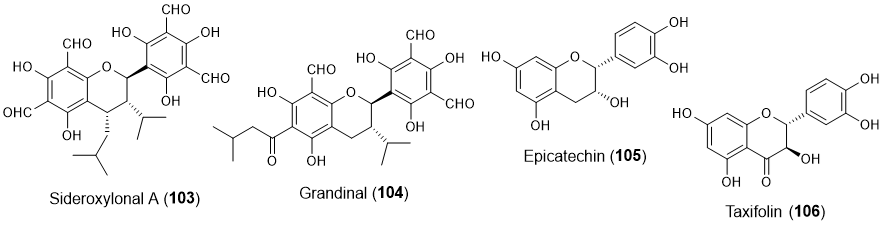
Figure 8. Structures of other classes of flavonoids with antifouling activity, including flavans 103-104, flavanol 105 and flavanonol 106. Flavonoids 105 and 106 were isolated from marine sources.
3. Conclusions
The phenomenon of marine biofouling is very complex, involving a wide diversity of marine organisms and depending on multiple variables, and, generally, the screening studies performed englobe a few or even just one biofouling species.
A total of 106 flavonoids with antifouling activity against macro- and microfouling organisms were compiled by chemical classes. Although some flavonoids were only evaluated for their activity against one single species of fouling organisms, namely the mussel M. galloprovincialis or the barnacle A. amphitrite, some compounds, including the flavonols kaempferol (63) and quercetin (64), or the flavone luteolin (85), displayed antifouling activity against several marine organisms, showing their potential to be effective in real scenarios of complex biofouling communities. Some flavonoids, namely the isoflavone genistein (100) and chalcones 4, 45, and 51–56, were incorporated into marine coatings, showing interesting antifouling potential in field assays.
Considering the need of obtaining environmentally safe antifoulants, ecotoxicological assessments are needed. In fact, only a few flavonoids were assessed for their ecotoxicological behavior against marine non-target species, namely chalcones 48–50 and 57–59 and flavones 75 and 95, which proved to be non-toxic against A. salina. In addition, studies of toxicity in human cell lines should be performed for the most promising antifouling compounds. Only chalcone 57 was assayed against one human cell line and the potential for toxicity was compared with a commercial biocide. Chalcone 57 was shown to be non-toxic, whereas the commercial biocide displayed toxicity to the human cell line at low concentrations.
Considering the feasible high-scale synthesis of flavonoids in the laboratory, allied with their high potential as antifoulants, this class of compounds could be considered for incorporation into antifouling paints.
This entry is adapted from the peer-reviewed paper 10.3390/md22020077
References
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Progress in Materials Science 2022, 124, 100889.
- Kyei, S.K.; Darko, G.; Akaranta, O. Chemistry and application of emerging ecofriendly antifouling paints: a review. Journal of Coatings Technology and Research 2020, 17, 315-332.
- Sarkar, P.K.; Pawar, S.S.; Rath, S.K.; Kandasubramanian, B. Anti-barnacle biofouling coatings for the protection of marine vessels: synthesis and progress. Environmental Science and Pollution Research 2022, 29, 26078-26112.
- Gomez-Banderas, J. Marine Natural Products: A Promising Source of Environmentally Friendly Antifouling Agents for the Maritime Industries. Frontiers in Marine Science 2022, 9.
- Poornima Vijayan, P.; Formela, K.; Saeb, M.R.; Chithra, P.G.; Thomas, S. Integration of antifouling properties into epoxy coatings: a review. Journal of Coatings Technology and Research 2022, 19, 269-284.
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environment International 2008, 34, 292-308.
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environmental Toxicology and Pharmacology 2018, 57, 115-130.
- Qian, P.-Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101-122.
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: chemistry and biological activities. Natural Product Research 2019, 33, 3260-3272.
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28.
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E., et al. Flavonoids: biological activities and therapeutic potential. Natural Product Research 2020, 34, 692-705.
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12.
- Yamashita, N.; Etoh, H.; Sakata, K.; Yagi, A.; Ina, H.; Ina, K. An Acylated Kaempferol Glucoside Isolated from Quercus dentata as a Repellent against the Blue Mussel Mytilus edulis. Agricultural and Biological Chemistry 1989, 53, 1383-1385.
- Sivakumar, P.M.; Prabhawathi, V.; Doble, M. Antibacterial activity and QSAR of chalcones against biofilm-producing bacteria isolated from marine waters. SAR and QSAR in Environmental Research 2010, 21, 247-263.
- Sivakumar, P.M.; Prabhawathi, V.; Doble, M. 2-Methoxy-2′,4′-dichloro chalcone as an antimicrofoulant against marine bacterial biofilm. Colloids and Surfaces B: Biointerfaces 2010, 81, 439-446.
- Almeida, J.R.; Moreira, J.; Pereira, D.; Pereira, S.; Antunes, J.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Correia-da-Silva, M.; Cidade, H. Potential of synthetic chalcone derivatives to prevent marine biofouling. Science of The Total Environment 2018, 643, 98-106.
- Sathicq, Á.; Paola, A.; Pérez, M.; Dallesandro, O.; García, M.; Roldán, J.P.; Romanelli, G.; Blustein, G. Furylchalcones as new potential marine antifoulants. International Biodeterioration & Biodegradation 2019, 143, 104730.
- Pereira, D.; Gonçalves, C.; Martins, B.T.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Almeida, J.R.; Correia-da-Silva, M.; Cidade, H. Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation. Marine Drugs 2021, 19.
- Singh, I.P.; Etoh, H.; Asai, E.; Kikuchi, K.; Ina, K.; Koyasu, K.; Terada, Y. Flavonoids and Stilbenes as Repellents against the Blue Mussel, Mytilus edulis galloprovincialis. Natural Product Sciences 1997, 3, 49-54.
- Zhou, X.; Zhang, Z.; Xu, Y.; Jin, C.; He, H.; Hao, X.; Qian, P.-Y. Flavone and isoflavone derivatives of terrestrial plants as larval settlement inhibitors of the barnacle Balanus amphitrite. Biofouling 2009, 25, 69-76.
- Kong, N.-N.; Fang, S.-T.; Liu, Y.; Wang, J.-H.; Yang, C.-Y.; Xia, C.-H. Flavonoids from the halophyte Apocynum venetum and their antifouling activities against marine biofilm-derived bacteria. Natural Product Research 2014, 28, 928-931.
- Gopikrishnan, V.; Radhakrishnan, M.; Shanmugasundaram, T.; Pazhanimurugan, R.; Balagurunathan, R. Antibiofouling potential of quercetin compound from marine-derived actinobacterium, Streptomyces fradiae PE7 and its characterization. Environmental Science and Pollution Research 2016, 23, 13832-13842.
- Almeida, J.R.; Correia-da-Silva, M.; Sousa, E.; Antunes, J.; Pinto, M.; Vasconcelos, V.; Cunha, I. Antifouling potential of Nature-inspired sulfated compounds. Scientific Reports 2017, 7, 42424.
- Haider, W.; Ma, J.; Hou, X.-M.; Wei, M.-Y.; Zheng, J.-Y.; Shao, C.-L. Natural Flavones and their Preliminary Structure–Antifouling Activity Relationship. Chemistry of Natural Compounds 2020, 56, 334-337.
- Jensen, P.R.; Jenkins, K.M.; Porter, D.; Fenical, W. Evidence that a New Antibiotic Flavone Glycoside Chemically Defends the Sea Grass Thalassia testudinumagainst Zoosporic Fungi. Applied and Environmental Microbiology 1998, 64, 1490-1496.
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Wang, B.-G. Antifeedant, antibacterial, and antilarval compounds from the South China Sea seagrass Enhalus acoroides. Botanica Marina 2008, 51, 441-447.
- Bao, J.; Sun, Y.-L.; Zhang, X.-Y.; Han, Z.; Gao, H.-C.; He, F.; Qian, P.-Y.; Qi, S.-H. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. The Journal of Antibiotics 2013, 66, 219-223.
- Guan, C.; Parrot, D.; Wiese, J.; Sönnichsen, F.D.; Saha, M.; Tasdemir, D.; Weinberger, F. Identification of rosmarinic acid and sulfated flavonoids as inhibitors of microfouling on the surface of eelgrass Zostera marina. Biofouling 2017, 33, 867-880.
- Yoshioka, A.; Etoh, H.; Yagi, A.; Sakata, K.; Ina, K. Isolation of Flavonoids and Cerebrosides from the Bark of Prunus jamasakura as Repellents against the Blue Mussel, Mytilus edulis. Agricultural and Biological Chemistry 1990, 54, 3355-3356.
- Feng, D.Q.; Ke, C.H.; Lu, C.Y.; Li, S.J. Herbal plants as a promising source of natural antifoulants: evidence from barnacle settlement inhibition. Biofouling 2009, 25, 181-190.
- Singh, I.P.; Takahashi, K.; Etoh, H. Potent Attachment-inhibiting and -promoting Substances for the Blue Mussel, Mytilus edulis galloprovincialis, from Two Species of Eucalyptus. Bioscience, Biotechnology, and Biochemistry 1996, 60, 1522-1523.
- Singh, I.P.; Hayakawa, R.; Etoh, H.; Takasaki, M.; Konoshima, T. Grandinal, a New Phloroglucinol Dimer from Eucalyptus grandis. Bioscience, Biotechnology, and Biochemistry 1997, 61, 921-923.
- Gopikrishnan, V.; Radhakrishnan, M.; Shanmugasundaram, T.; Ramakodi, M.P.; Balagurunathan, R. Isolation, characterization and identification of antibiofouling metabolite from mangrove derived Streptomyces sampsonii PM33. Scientific Reports 2019, 9, 12975.
