Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Synucleinopathies represent a diverse set of pathologies with significant morbidity and mortality.
- synucleinopathies
- Parkinson’s disease
- multiple system atrophy
- dementia with Lewy bodies
- deep brain stimulation
- focused ultrasound
- gene therapy
- surgical techniques
1. Introduction
The three members of the α-synucleinopathy family—Parkinson’s disease (PD), multiple system atrophy (MSA), and dementia with Lewy bodies (DLB)—are some of the most common and costly neurodegenerative diseases affecting neurosurgical patients [1]. Parkinson’s disease alone affects more than one million individuals in the United States [1,2,3]. In addition to the untold emotional burden, these afflictions place an economic strain upon the nation currently exceeding USD 50 billion per annum [4]. Considering Parkinson’s disease alone is projected to double in incidence rate by 2030 due to an aging population, it is essential to establish optimal management protocols for α-synucleinopathies [5].
2. Deep Brain Stimulation (DBS)
2.1. DBS and α-Synuclein in PD
Despite promising pharmacological treatment options, α-synucleinopathies can prove to be extremely difficult to manage medically. For one, prolonged levodopa treatment is associated with significant side effects in PD such as dyskinesias and motor fluctuations between the on and off state [40]. Unfortunately, due to the progressive nature of the disease, dose escalation is an eventuality in most patients [41]. Deep brain stimulation (DBS) offers a promising surgical option where a high frequency electrode is implanted into a target structure in the basal ganglia or thalamus such as the globus pallidus internus (GPi), subthalamic nucleus (STN) or ventral intermediate nucleus (VIM) [42]. High frequency electrical stimulation is then delivered from the DBS electrode to normalize the pathologically exaggerated basal ganglia bursting activity seen in PD [43]. There is a potential for significant stimulation induced side effects such as paresthesia, involuntary motor contractions, speech impairment, and mood changes. To minimize these side effects, parameters of stimulation such as its amplitude and directionality relative to the DBS electrode are controlled in programming sessions in the weeks following initial implantation. DBS therapy often leads to significant reduction in medication regimens and significant prolongation of “on” medication periods. In one meta-analysis of 22 studies, L-dopa regimens were reduced over 50%, with dyskinesias reduced around 70%, and 70% reduction in “off” periods [44].
Despite the ability of DBS therapy to significantly improve quality of life, it is widely believed that it cannot stop or reverse the effects of α-synuclein-mediated neurodegeneration in PD. In fact, disease progression with loss of dopaminergic neurons is thought to occur rapidly, within 4 years [45]. Nevertheless, several recent works have expanded knowledge concerning the effect of DBS on disease progression, though there remains considerable debate as to whether long term DBS stimulation confers a neuroprotective effect. In one study, rats were induced to overexpress α-synuclein using intranigral injections of an adeno-associated viral (AAV) vector and were subsequently implanted with STN DBS [46]. Limb use by the animals was observed at baseline as well as after electrode implantation and after electrode stimulation for a period of 26 days and was compared to mice that were implanted but did not receive stimulation. Of note, though there was impaired contralateral limb use following the α-syn vector injection in all rats, there was no difference in limb use between stimulation and nonstimulation groups. Neurodegenerative changes were assessed using tyrosine hydroxylase staining (a marker of dopaminergic neurons), which also did not differ between stimulation and nonstimulation groups. This result suggests that DBS stimulation does not protect against the impairment nor the neurodegenerative changes that accompany α-synuclein accumulation [46]. Despite this, another very similar study using an AAV induced α-synuclein overexpression rat model implanted with STN DBS reported different findings. In particular, results of this study suggest that motor performance after 3 weeks of stimulation was significantly improved compared to rats in the nonstimulation group, even with stimulation turned off during motor testing. Tyrosine hydroxylase was also significantly increased in the stimulation group [47].
In addition, beta oscillations are a significant pathologic alteration observed in PD and are linked to some of its symptoms [48]. Recent evidence suggests that STN DBS suppresses these pathological beta oscillations in an AAV induced α-synuclein overexpression rat model, even 2 weeks after stimulation [49]. Other studies have also demonstrated the possible neuroprotective effects of DBS. Specifically, brain derived neurotrophic factor (BDNF) has come under interest recently for its roles in plasticity, neurogenesis, and neuroprotection [50]. In one study of rats injected with preformed fibrils of α-synuclein, though STN DBS did not impact α-synuclein deposition or total BDNF, rats in the stimulation group displayed restoration of striatal BDNF when compared to those in the nonstimulation group [51]. Furthermore, DBS may exert a neuroprotective effect through the inhibition of neuroinflammatory cytokines and pathways [52] and the modulation of synaptic plasticity [53]. Taken together, the findings related to α-synuclein deposition in PD reiterate both sides of thought related to DBS-induced neuroprotection (Figure 1).
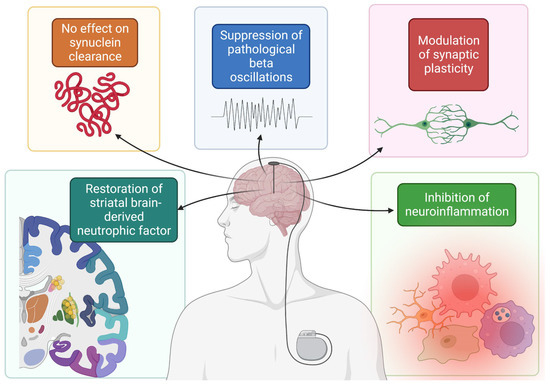
Figure 1. Summary of mechanisms by which DBS possibly confers a neuroprotective effect. Figure created with BioRender.com, accessed on 19 September 2022.
One central regulator of α-synuclein accumulation is autophagy. Rapamycin, for instance, stimulates autophagy and improves clearance of α-synuclein [54]. Notably, derailments in α-synuclein and autophagy in the perioperative environment can lead to pathology. In turn, an important consideration in the surgical treatment of synucleinopathies is the effect of anesthetic on α-synuclein, which was explored in a recent preclinical study. In this study, rats were anesthetized with propofol for 4 h and were assessed for neurobehavioral and cognitive deficits and hippocampal α-synuclein deposition. Along with elevated hippocampal α-synuclein deposition and impaired autophagy, 4 h of propofol induced worse performance on the Morris water maze test and shorter freezing times in the freezing conditioning test [55]. These results have important implications in the surgical management of PD with DBS, where electrode implantation being conducted awake or under general anesthetic is highly center dependent.
2.2. DBS in DLB and MSA
Despite the abundance of work related to DBS and PD, there is a relative paucity of evidence regarding the indications and benefits related to DBS use in the remaining synucleinopathies. The risk of cognitive decline is a central issue in DBS, making preoperative neuropsychiatric evaluation of paramount importance. In one study of 60 patients treated with STN DBS, executive functioning was significantly reduced when compared to a control group receiving standard medical therapy but no DBS [56]. Risk factors for cognitive decline following DBS included age and larger preoperative medication doses. Given that patients with DLB and MSA are already at significant risk for dysfunctional cognition, this possible adverse effect should be carefully considered in the context of each patient being evaluated for DBS therapy. Nevertheless, there has been some investigation into its use in DLB and MSA. In one randomized, double blind crossover study of 6 DLB patients implanted with bilateral nucleus basalis of Meynert (NBM) electrodes, there was no difference between stimulation and control conditions in a variety of cognitive tasks. Importantly, the procedure was well-tolerated and there was a positive impact on neuropsychiatric inventory (NPI) scores in that study [57]. However, in another more recent phase 1 study of six patients with bilateral NBM DBS, some cognitive decline occurred following electrode implantation [58]. MSA is also thought to be largely unresponsive to DBS and may be an underlying explanation for why neuromodulation fails in some patients with PD [59,60]. In one recent systematic review of 12 studies representing 22 patients with MSA, the majority were treated with bilateral STN electrodes (n = 18) or bilateral GPi (n = 3) [61]. At a median follow-up of 12 months, though subjective improvements in bradykinesia, gait, and rigidity were accompanied by a more than 12-point reduction in UPDRS-III score, a significant 23% of patients displayed neurobehavioral or neurocognitive side effects following DBS implantation. In MSA patients, who are particularly at risk for cognitive impairments [62], this represents a significant barrier to the safety and tolerability of DBS in MSA61. Furthermore, this systematic review also included 12 patients with DLB treated with bilateral NBM DBS. Again, no significant improvements in quality of life nor cognitive measurements were appreciated in this study [61]. Taken together, this suggests that the surgical indications for DBS therapy in MSA and DLB are still poorly understood and require further investigation.
3. Focused Ultrasound
Focused ultrasound (FUS) therapy is an actively studied alternative to current standard treatments involving open surgeries [63]. The primary benefit of FUS therapy is its ability to induce biological effects on deeper target tissue without damaging surrounding tissues [63]. FUS therapy is achieved using a piezoelectric ultrasound transducer to deliver a FUS beam and is guided using imaging modalities such as a traditional ultrasound or Magnetic Resonance Imaging (MRI) [64] to provide simultaneous monitoring of tissue effects [63]. The FUS beam is steered with precision by mechanically manipulating the transducer [64], and the spatial specificity of the beam and depth of its effects can be parametrized by varying the delivered sonication frequency and intensity [65]. At high intensity, the delivered FUS beam induces two main effects on target tissue: thermoablation and cavitation. Thermoablation results from tissue absorption of the beam energy, which rapidly increases the tissue temperature to irreversibly cytotoxic levels [66]. FUS mechanically induces cavitation, or the creation of gas cavities, in tissue by expanding and compressing the tissue as it travels through it [66]. These effects are purposely leveraged in clinical treatments to target varying tissues of interest as an alternative approach to surgery. Previous research demonstrates the use of FUS thermoablation to treat uterine fibroids [67], advanced stage renal malignancy [68], and primary bone tumors [69], and thus may offer a potential alternative to contemporary invasive surgeries. FUS has emerged as a new modality for treating movement disorders—such as essential tremor and PD—through noninvasive lesioning. Additionally, FUS therapy does not require hardware placement, such as an electrode, minimizing the risk of perioperative infection. However, FUS therapy faces limitations such as attenuation from overlying tissue, sensitivity to patient movement, possible treatment times of up to several hours, and the fact that lesioning is permanent and irreversible [64,70].
4. Other Approaches in the Management of MSA and DLB
Though surgical interventions are poorly understood in the context of MSA and DLB, several promising frontiers of treatment have recently come to light. For example, in MSA, autonomic dysfunction remains a critical issue, characterized by progressive sympathetic failure, namely in the form of orthostatic hypotension [91]. This orthostatic hypotension can lead to a number of downstream sequelae, including cerebral hypoperfusion and increased risk for falls from resulting dizziness [91]. In fact, autonomic dysregulation is a predictor of worse outcomes in MSA and faster disease progression [92]. One recent study is poised to target this debilitating comorbidity of MSA [93]. This study describes the implantation of an epidural thoracic cord stimulator in a 48-year-old woman with progressive sympathetic dysfunction with resultant orthostatic hypotension. This stimulator is paired with an accelerometer which detects when the patient stands up and controls the delivery of stimulation to the thoracic cord. With the stimulator off, an 85 mmHg drop in systolic blood pressure was observed within 3 min of tilting the patient upright. This is compared to an 85 mmHg drop in systolic blood pressure occurring over 10 min after the stimulation was turned on [93]. Though this study lacks the power of a large clinical trial, it represents a promising treatment option for a very debilitating comorbidity of MSA.
In DLB, pathology stems in part from the deposition of extracellular synuclein, which leads to dysfunctional synaptic transmission and plasticity [33]. Transcranial direct current stimulation (tDCS) may play a role in modulating cortical excitability and is thought to induce changes in synaptic plasticity [94]. One recent double-blind clinical trial tested the efficacy of 10 days of tDCS sessions in improving the cognitive and psychiatric assessments of 11 DLB patients versus sham tDCS. Though there were no adverse effects from the treatment, no significant cognitive or psychiatric differences were found between the groups [87].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102657
This entry is offline, you can click here to edit this entry!
