Produced by photosynthesis, oxygen (O2) is a fundamentally important gas in biological systems, playing roles as a terminal electron receptor in respiration and in host defence through the creation of reactive oxygen species (ROS). Hydrogen (H2) plays a role in metabolism for some organisms, such as at thermal vents and in the gut environment, but has a role in controlling growth and development, and in disease states, both in plants and animals.
1. Introduction
Since its discovery in the latter part of the 18th century [
1], there has been an interest in how oxygen (O
2) has roles in biological systems, including its detection [
2] and toxicity [
3]. O
2 makes up approximately 21% of the atmosphere and is essential for aerobic life. O
2 is produced by photosynthesis as a by-product of plant physiology and it is used in respiratory processes, being the terminal electron acceptor for the mitochondrial electron transport chain (Complex IV converting O
2 to water). Oxygen has many other uses in normal physiology, too, not least being the starting point of reactive oxygen species (ROS), as outlined in
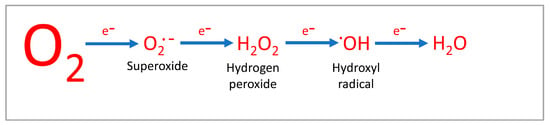
Figure 1.
Figure 1. Oxygen can be reduced to reactive oxygen species (ROS), such as superoxide anions (O2•−), hydrogen peroxide (H2O2) and the hydroxyl radical (•OH). In mitochondrial respiration, O2 is converted to water by a 4-electron reaction.
Superoxide (O2•−) is regarded as the cardinal ROS as its formation is known to give rise to other ROS including the hydroxyl radical (•OH), and also RNS such as peroxynitrite (ONOO−). The dismutation of O2•− by superoxide dismutase (SOD) gives rise to hydrogen peroxide (H2O2), which can generate •OH through downstream reactions noted below in Equations (1) and (2):
The further reduction of
•OH yields water, and it is the four-electron reduction of O
2 by mitochondrial Complex IV which aims to avoid ROS generation. Such reactive molecules are chemically relatively unstable, as many carry unpaired electrons in the outer orbit which have a strong affinity for hydrogen atoms. This can lead to proton extraction from vital biomolecules such as protective lipid membranes, thereby destabilising their structure and altering functionality. ROS/RNS are also able to affect fundamental processes including genetic transcription and mitochondrial activity through the modification of essential proteins and protective membranes and therefore have a significant role in cellular homeostasis. Several excellent reviews on ROS function are available [
4,
5,
6].
Hydrogen was also discovered in the 18th century, and since then has had a rather on-and-off appearance in biological research [
7,
8]. However, hydrogen gas has been coming to prominence in the scientific literature more recently, especially since the publication of a
Nature Medicine paper in 2007 [
9], not only as an alternative source of renewable energy but also as a health and lifestyle supplement for humans and animals with potential uses in surgical [
10], recuperative [
11] and geriatric medicine [
12]. Research also suggests incorporating H
2 into the agri-food industry, where it can be used in many ways and can enhance stress tolerance [
13,
14] and act as a growth stimulant [
15] or as a preservative for plants and food products [
16,
17], for example.
Table 1 and
Table 2 outline the significant effects molecular hydrogen can have in both animals and plants.
Table 1. Examples of the effects seen in animals after molecular hydrogen treatment. HRS: Hydrogen-rich saline; HRW: Hydrogen-rich water MDA: Malondialdehyde; SOD: superoxide dismutase; Nrf-2: nuclear factor erythroid 2-related factor 2; HO-1: haem oxidase-1; NQO-1: NAD (P)H quinone oxidoreductase 1; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor; RBC: red blood cell; WBC: white blood cell; COX-2: cyclooxygenase-2; 8-oxo-dG: 8-Oxo-2′-deoxyguanosine; CLDN3: Claudin 3.
Table 2. Examples of the effects seen in plants after molecular hydrogen treatment. APX: ascorbate peroxidase; CAT: catalase; POD: peroxide; SOD: superoxide dismutase.
Organisms can be exposed to H
2 in a variety of ways, with several species containing hydrogenase enzymes, which will produce H
2 [
33]. Hydrogenase enzymes, some of which are inhibited by O
2 (e.g., group IV NiFe hydrogenases [
34]), are responsible for catalysing the reversible oxidation/reduction of H
2 (H
2 ↔ 2H
+ + e
−). Such enzymes are found in all single-celled and many multicellular organisms (e.g., fungi, plants [
35]) and can be categorised into specific phylogenic groups, namely iron only, iron-iron and nickel-iron (Fe, Fe-Fe and NiFe, accordingly). On the other hand, other organisms host an array of H
2-producing microflora in the gastrointestinal tract. Hydrogen can also be naturally occurring, for example in thermal vents [
36] and other natural sources [
37]. As with O
2, organisms can also be treated with molecular hydrogen which can be applied directly as a gas, or as hydrogen-enriched water (hydrogen-rich water: HRW: [
38]), perhaps in the form of nanobubbles (hydrogen nanobubble water (HNW)). Furthermore, organisms can be exposed to O
2 and H
2 at the same time in the form of oxygen/hydrogenated solutions and as a stoichiometric mixture of oxyhydrogen gas (66% H
2/33% O
2) formed through the electrolysis of water.
Although the role of action of O2 in cells is well established, the way H2 effects cells is still unclear under many circumstances. As listed in Table 3, there are several mechanisms possible, but it is unlikely that a single action would be able to account for all the cellular responses reported. H2 was originally thought to be acting as an antioxidant, with many of its effects from scavenging hydroxyl radicals (•OH) but having little or no effect on several significant small reactive signalling molecules such as superoxide (O2•−), H2O2 or nitric oxide (NO). However, as Table 3 exemplifies, several other mechanisms have been proposed, or may at least be possible, and multiple mechanisms are likely to be invoked simultaneously upon rising H2 concentrations. Several of these mechanisms may involve O2, and it seems timely to discuss what any possible ramifications of this may be.
Table 3. Some of the possible mechanisms of the action of H2 in cells and tissues.
Water (H
2O), composed of hydrogen and oxygen, is a universal biological solvent and is essential for the emergence and continuation of carbon-based life [
49]. H
2O is formed through the thermal catalysis of O
2 and hydrogen (H
2) gases, (reaction 2H
2 + O
2 → 2H
2O), but of importance here is that it can also be split to yield O
2 and H
2 in a ratio of 1:2. An inexpensive and relatively simple way to generate H
2 is by electro-hydrolysis, with the H
2 being exploited and the O
2 being vented to waste.
2. Solubility of Oxygen and Hydrogen in Water
When using either O
2 or H
2 in solution, the solubility of the gas has to be considered. The solubility of O
2 in pure water is reported to be between 1.18 × 10
−3 and 1.25 × 10
−3 mol dm
−3 (at 25 °C and 1.0 atm of O
2 pressure) [
51]. For H
2, the solubility under similar conditions, ~1 atm and 25 °C, is approximately 0.8 × 10
−3 mol dm
−3.
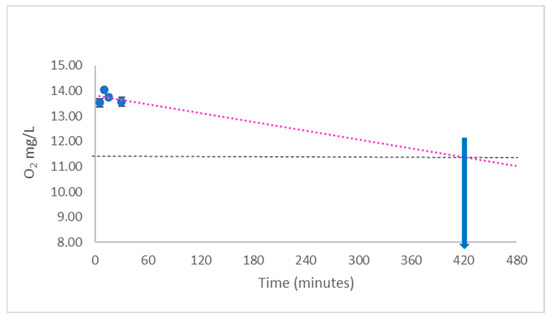
If water is equilibrated with air, the O2 concentration would be predicted to be approximately 2.56 × 10−4 mol dm−3, whilst the concentration of H2 would be predicted to be negligible—there is little atmospheric H2 to dissolve at ground level. If the concentration of O2 or H2 is increased in water, one of the questions which should be asked is for how long does that elevated concentration last? As can be seen in Figure 2, an elevated level of O2 in solution starts to decline and if the regression line is extrapolated, compared to the baseline concentration, it gives a half-life of elevated oxygen in solution of approximately 420 min. Therefore, if such a solution is going to be used as a treatment for any organism, be it a plant or animal, the loss of oxygen gas with time needs to be considered—it would be suggested that such solutions are made shortly after being prepared.
Figure 2. Estimation of the O2 concentration in solution. On extrapolation, this gives the half-life of concentration elevation. The solution was bubbled with oxyhydrogen solution 450 mL/min for 30 min (HydroVitality™, Wakefield, UK). O2 was measured with a Clark-type electrode (Hanna Instruments, Bedfordshire, UK. Cat. #H198198). The red dotted line is an extrapolation, whilst the horizonal line represents the half-way point of increase. The arrow indicates an approximate half-life. Data representative of 3 repeats +/− SEM.
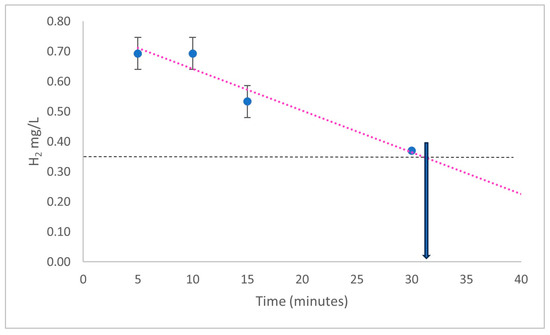
Similarly, if hydrogen gas in solution is elevated, it will rapidly re-enter the gaseous phase and be lost. Again, representative data are shown (Figure 3), showing that under the conditions used, the half-life of the H2 in solution is only approximately 32 min (Figure 3). Therefore, long-term storage of such a solution before use is not an option unless kept in a sealed container with little head space. Of critical importance here, such measurements of O2 and H2 in solution should be carried out by researchers using their equipment and solutions before being used in a biological setting.
Figure 3. Estimation of the H2 concentration in solution after bubbling with oxyhydrogen gas 450 mL/min for 30 min (HydroVitality™, Wakefield, UK). H2 was measured with a methylene blue-based assay system (H2Blue, H2 Sciences Inc., Henderson, NV, USA). The horizontal dotted line is halfway between the maximal concentration measured and the minimal concentration expected when the solution is equilibrated with atmospheric air. The red dotted line is an extrapolation, whilst the horizonal line represents the half-way point of increase. The arrow indicates an approximate half-life Data representative of 3 repeats +/− SEM.
In a very recent study [
52] on the proton exchange membrane (PEM) electrolysis of water (a method that infuses only H
2 into aqueous solutions) from different sources, the increased H
2 in each solution was shown to have a half-life of between approximately 60 and 140 min, which is longer than found in our studies involving alkaline electrolysis that produces and infuses both H
2 and O
2 (~32 min half-life). Interestingly, the results are widely different depending on the source of water used. The shortest half-life was with what the authors describe as “Holy Spring” water, with the longest being tap water. The study also found that the highest H
2 concentration they could obtain was approximately 1.3 ppm. pH rose by about 1 unit over 70 min of electrolysis, and the oxidation-reduction potential (ORP) dropped significantly, from approximately +200 mV to −500 mV. Such work on different waters highlights the need to be careful about knowing what is dissolved in solution and to measure exact working concentrations when reporting data with dissolved gases.
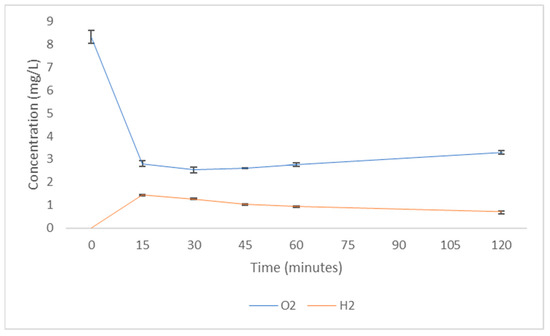
Lastly, it is likely the dissolving of one gas in an aqueous solution (whether pure water, saline or buffer) may affect the presence of other gases. For example, a representative example is shown in Figure 4. Here, it can be seen that the concentration of O2 in solution drops significantly whilst the magnesium-based tablet produces H2 (by the Mg catalysis of water hydrolysis), but then the O2 levels are slowly restored as the oxygen is absorbed from, and hydrogen released into, the atmosphere. Creating a fresh H2 solution in such a fashion may make a relatively anaerobic solution, and should the creation of such an anaerobic solution not be considered as a mode of action when such solutions are used in agricultural and biomedical research, or as therapies in a medical/sports medicine arena?
Figure 4. Concentration of O2 and H2 in solution. H2 was produced using a magnesium-based tablet (Drink HRW, Oxnard, USA). The tablet was dropped into deionised water (15 MΩ∙cm2) in a non-sealed conical flask (500 mL). H2 was measured with a methylene blue-based assay system (H2Blue, H2 Sciences Inc., Henderson, NV, USA). O2 was measured using a Clark-type electrode (Hanna Instruments, Bedfordshire, UK. Cat. #H198198). Data are mean 3 repeats +/− SEM.
This entry is adapted from the peer-reviewed paper 10.3390/oxygen4010003