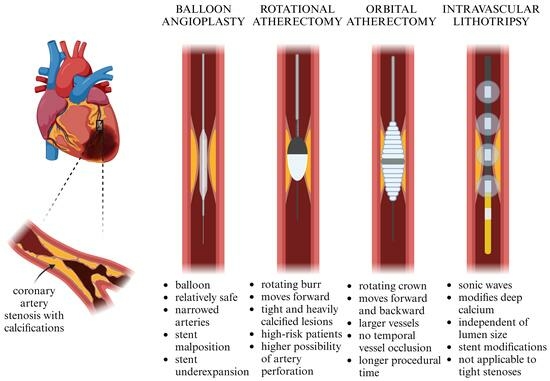
In order to improve the percutaneous treatment of coronary artery calcifications (CAC) before stent implantation, methods such as rotational atherectomy (RA), orbital atherectomy (OA), and coronary intravascular lithotripsy (IVL) were invented. These techniques use different mechanisms of action and therefore have various short- and long-term outcomes. IVL employs sonic waves to modify CAC, whereas RA and OA use a rapidly rotating burr or crown. These methods have specific advantages and limitations, regarding their cost-efficiency, the movement of the device, their usefulness given the individual anatomy of both the lesion and the vessel, and the risk of specified complications.
- rotational atherectomy
- orbital atherectomy
- intravascular lithotripsy
- coronary artery calcifications
- percutaneous coronary interventions
- IVUS
- OCT
1. Mechanism of Action
In order to avoid poor procedural outcomes and an increased risk of significant adverse cardiovascular events, procedures like debulking the plaques before stent implantation have been implemented (Figure 1).
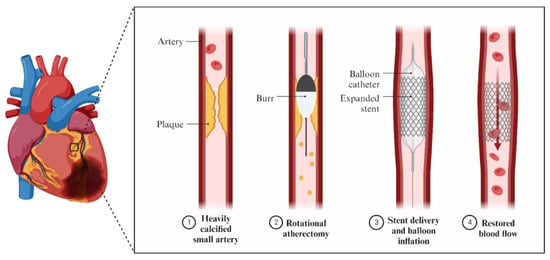
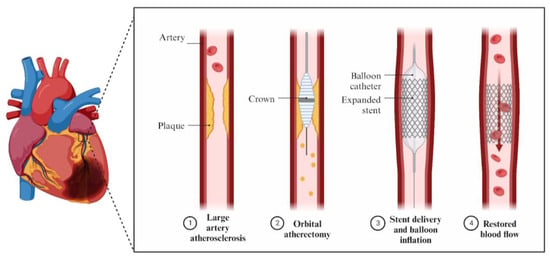
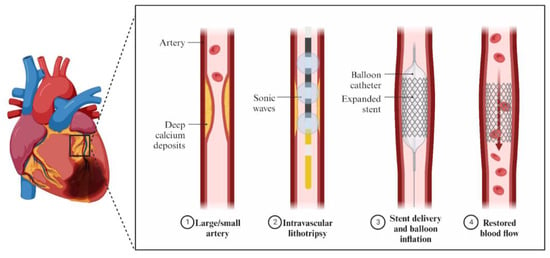
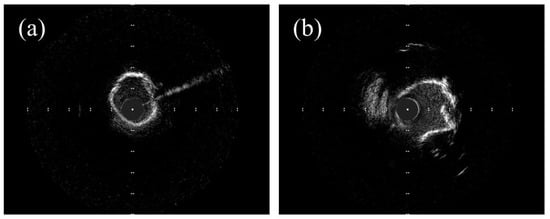
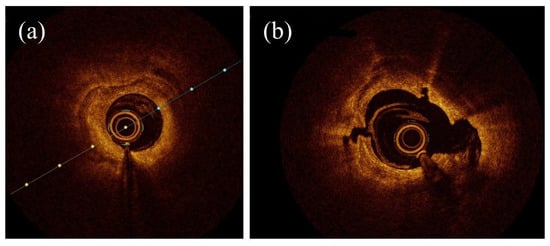
2. Intravascular Imaging in RA, OA, and IVL
| IVUS | Diagnosis of intermediate stenosis of the left coronary artery trunk |
| IVUS/OCT | Optimization of stent implantation procedures in native arteries |
| Coronary artery recanalization procedures (guidewire position assessment, true/false lumen navigation) | |
| Studies on the progression/regression of atherosclerosis | |
| IVUS > OCT | Optimizing the left coronary artery trunk angioplasty procedure |
| Imaging for spontaneous coronary artery dissection | |
| Vasculopathy after heart transplantation | |
| OCT > IVUS | Optimizing revascularization in patients with current coronary artery calcifications |
| Intracoronary imaging for suspected acute coronary syndrome | |
| Diagnosis of the causes of stent implantation failure | |
| Diagnosis of neo-atherosclerosis |
2.1. OCT Assessment in RA, OA, and IVL
2.2. OCT vs. IVUS in RA, OA, and IVL
3. Effectiveness
| Category | Parameter | RA | OA | IVL |
|---|---|---|---|---|
| Mechanism of action | Device | Rotating burr (140,000–160,000 rpm) |
Rotating crown (80,000–120,000 rpm) |
Emits sonic waves (80–120 impulses) |
| Independent of lumen size | − | − | + | |
| Modifies deep calcium | − | +/− | + | |
| Temporal vessel occlusion | + | − | ++ | |
| Tight stenosis | + | + | − | |
| Wire bias | + | + | − | |
| Modifies noncalcified lesions | − | + | − | |
| Treatment of in-stent restenosis | − | − | + | |
| Periprocedural complications | Wire entrapment | ++ | + | − |
| No-/slow-flow risk | 6–15% | 0.9% | None reported | |
| Dissection | Lower risk | Higher risk | Rare | |
| Perforation | Lower risk | Higher risk | Rare | |
| Effectiveness | Procedural success (stent delivery) |
92.5%, 98% | 97.7% | 92.4% |
| Short-term outcomes | 30-day MACE | 5% | 10.4% | 7.2% |
| Long-term outcomes | 1-year MACE | 15% | 14.4% | 13.2% |
“+”—applicable/present; “−”— no applicable; if more than one sign is used it is associated with feature intensity.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12237246
References
- Pietzsch, J.B.; Geisler, B.P.; Ikeno, F. Cost-Effectiveness of Orbital Atherectomy Compared to Rotational Atherectomy in Treating Patients with Severely Calcified Coronary Artery Lesions in Japan. Cardiovasc. Interv. Ther. 2018, 33, 328.
- Sharma, S.K.; Tomey, M.I.; Teirstein, P.S.; Kini, A.S.; Reitman, A.B.; Lee, A.C.; Généreux, P.; Chambers, J.W.; Grines, C.L.; Himmelstein, S.I.; et al. North American Expert Review of Rotational Atherectomy. Circ. Cardiovasc. Interv. 2019, 12, e007448.
- Rotational vs. Orbital Atherectomy: How to Choose?|SCAI. Available online: https://scai.org/rotational-vs-orbital-atherectomy-how-choose?fbclid=IwAR2ctK1nNyVGILINLBXe6pSjS3XYaKgTNG3vTX4RtdemSIamBJJAdxqbSXc (accessed on 25 February 2023).
- Tomasiewicz, B.; Kubler, P.; Zimoch, W.; Kosowski, M.; Wańha, W.; Ładziński, S.; Rakotoarison, O.; Ochała, A.; Wojakowski, W.; Reczuch, K. Acute Angulation and Sequential Lesion Increase the Risk of Rotational Atherectomy Failure. Circ. J. 2021, 85, 867–876.
- Kübler, P.; Zimoch, W.; Kosowski, M.; Tomasiewicz, B.; Telichowski, A.; Reczuch, K. Acute Coronary Syndrome—Still a Valid Contraindication to Perform Rotational Atherectomy? Early and One-Year Outcomes. J. Cardiol. 2018, 71, 382–388.
- Reisman, M.; Shuman, B.J.; Harms, V. Analysis of Heat Generation during Rotational Atherectomy Using Different Operational Techniques. Cathet. Cardiovasc. Diagn. 1998, 44, 453–455.
- Dhall, A.; Koshy, S.; Chouhan, N. Percutaneous Intervention of Calcific Coronary Stenosis. Available online: https://www.researchgate.net/publication/278786408_Percutaneous_Intervention_of_Calcific_Coronary_Stenosis (accessed on 2 October 2023).
- Gupta, T.; Weinreich, M.; Greenberg, M.; Colombo, A.; Latib, A. Rotational Atherectomy: A Contemporary Appraisal. Interv. Cardiol. Rev. 2019, 14, 182.
- Barbato, E.; Carrié, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European Expert Consensus on Rotational Atherectomy. EuroIntervention 2015, 11, 30–36.
- Sotomi, Y.; Shlofmitz, R.A.; Colombo, A.; Serruys, P.W.; Onuma, Y. Patient Selection and Procedural Considerations for Coronary Orbital Atherectomy System. Interv. Cardiol. Rev. 2016, 11, 33.
- Dini, C.S.; Nardi, G.; Ristalli, F.; Mattesini, A.; Hamiti, B.; Di Mario, C. Contemporary Approach to Heavily Calcified Coronary Lesions. Interv. Cardiol. Rev. 2019, 14, 154–163.
- Redfors, B.; Sharma, S.K.; Saito, S.; Kini, A.S.; Lee, A.C.; Moses, J.W.; Ali, Z.A.; Feldman, R.L.; Bhatheja, R.; Stone, G.W. Novel Micro Crown Orbital Atherectomy for Severe Lesion Calcification. Circ. Cardiovasc. Interv. 2020, 13, e008993.
- Rola, P.; Kulczycki, J.J.; Barycki, M.; Włodarczak, S.; Furtan, Ł.; Kędzierska, M.; Giniewicz, K.; Doroszko, A.; Lesiak, M.; Włodarczak, A. Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes. J. Clin. Med. 2023, 12, 4025.
- Shlofmitz, E.; Martinsen, B.J.; Lee, M.; Rao, S.V.; Généreux, P.; Higgins, J.; Chambers, J.W.; Kirtane, A.J.; Brilakis, E.S.; Kandzari, D.E.; et al. Orbital Atherectomy for the Treatment of Severely Calcified Coronary Lesions: Evidence, Technique, and Best Practices. Expert Rev. Med. Devices 2017, 14, 867–879.
- Shlofmitz, E.; Chambers, J.; Moses, J.W.; Martinsen, B.; Meraj, P.; Jauhar, R.; Shlofmitz, R. TCT-389 Temporary Pacemaker Placement Incidence with the Diamondback 360® Coronary Orbital Atherectomy System Compared to Rotational Atherectomy. J. Am. Coll. Cardiol. 2015, 66, B157.
- Wong, J.J.; Umapathy, S.; Keh, Y.S.; Lau, Y.H.; Yap, J.; Idu, M.; Chin, C.Y.; Fam, J.M.; Liew, B.W.; Chin, C.T.; et al. Coronary Intravascular Lithotripsy Versus Rotational Atherectomy in an Asian Population: Clinical Outcomes in Real-World Patients. Korean Circ. J. 2022, 52, 288–300.
- Okamoto, N.; Egami, Y.; Nohara, H.; Kawanami, S.; Sugae, H.; Kawamura, A.; Ukita, K.; Matsuhiro, Y.; Nakamura, H.; Yasumoto, K.; et al. Direct Comparison of Rotational vs Orbital Atherectomy for Calcified Lesions Guided by Optical Coherence Tomography. JACC Cardiovasc. Interv. 2023, 16, 2125–2136.
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646.
- Wańha, W.; Tomaniak, M.; Wańczura, P.; Bil, J.; Januszek, R.; Wolny, R.; Opolski, M.P.; Kuźma, Ł.; Janas, A.; Figatowski, T.; et al. Intravascular Lithotripsy for the Treatment of Stent Underexpansion: The Multicenter IVL-DRAGON Registry. J. Clin. Med. 2022, 11, 1779.
- Honton, B.; Monsegu, J. Best Practice in Intravascular Lithotripsy. Interv. Cardiol. Rev. Res. Resour. 2022, 17, e02.
- Kimball, B.P.; Bui, S.; Cohen, E.A.; Carere, R.G.; Adelman, A.G. Early Experience with Directional Coronary Atherectomy: Documentation of the Learning Curve. Can. J. Cardiol. 1993, 9, 177–185.
- Ali, Z.A.; Maehara, A.; Généreux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical Coherence Tomography Compared with Intravascular Ultrasound and with Angiography to Guide Coronary Stent Implantation (ILUMIEN III: OPTIMIZE PCI): A Randomised Controlled Trial. Lancet 2016, 388, 2618–2628.
- Kaul, A.; Dhalla, P.S.; Bapatla, A.; Khalid, R.; Garcia, J.; Armenta-Quiroga, A.S.; Khan, S. Current Treatment Modalities for Calcified Coronary Artery Disease: A Review Article Comparing Novel Intravascular Lithotripsy and Traditional Rotational Atherectomy. Cureus 2020, 12, e10922.
- Mattesini, A.; Nardi, G.; Martellini, A.; Sorini Dini, C.; Hamiti, B.; Stolcova, M.; Meucci, F.; Di Mario, C. Intravascular Imaging to Guide Lithotripsy in Concentric and Eccentric Calcific Coronary Lesions. Cardiovasc. Revascularization Med. 2020, 21, 1099–1105.
- Shlofmitz, R.A.; Galougahi, K.K.; Jeremias, A.; Shlofmitz, E.; Thomas, S.V.; Ali, Z.A. Calcium Modification in Percutaneous Coronary Interventions. Interv. Cardiol. Clin. 2022, 11, 373–381.
- Lee, M.S.; Shlofmitz, E.; Kong, J.; Lluri, G.; Srivastava, P.K.; Shlofmitz, R. Impact of the Use of Intravascular Imaging on Patients Who Underwent Orbital Atherectomy. J. Invasive Cardiol. 2018, 30, 77–80.
- Sandhyavenu, H.; Ullah, W.; Badu, I.; Zghouzi, M.; Baqal, O.; Ali, M.; Mir, T.; Minhas, A.M.K.; Johnson, D.; Virani, S.S.; et al. Outcomes of Intravascular Imaging in Orbital Atherectomy; Insight from the National Readmissions Database. Curr. Probl. Cardiol. 2023, 48, 101475.
- Maehara, A.; Matsumura, M.; Ali, Z.A.; Mintz, G.S.; Stone, G.W. IVUS-Guided Versus OCT-Guided Coronary Stent Implantation. JACC Cardiovasc. Imaging 2017, 10, 1487–1503.
- Peng, C.; Wu, H.; Kim, S.; Dai, X.; Jiang, X. Recent Advances in Transducers for Intravascular Ultrasound (IVUS) Imaging. Sensors 2021, 21, 3540.
- de Donato, G.; Pasqui, E.; Alba, G.; Giannace, G.; Panzano, C.; Cappelli, A.; Setacci, C.; Palasciano, G. Clinical Considerations and Recommendations for OCT-Guided Carotid Artery Stenting. Expert Rev. Cardiovasc. Ther. 2020, 18, 219–229.
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030166373.
- Cortese, B.; Piraino, D.; Gentile, D.; Onea, H.; Lazar, L. Intravascular Imaging for Left Main Stem Assessment: An Update on the Most Recent Clinical Data. Catheter. Cardiovasc. Interv. 2022, 100, 1220–1228.
- Moulias, A.; Koros, R.; Papageorgiou, A.; Patrinos, P.; Spyropoulou, P.; Vakka, A.; Bozika, M.; Vasilagkos, G.; Apostolos, A.; Nastouli, K.-M.; et al. OCT Guidance in Bifurcation Percutaneous Coronary Intervention. Rev. Cardiovasc. Med. 2023, 24, 88.
- Pawlowski, T.; Legutko, J.; Kochman, J.; Roleder, T.; Pregowski, J.; Chmielak, Z.; Kubica, J.; Ochala, A.; Parma, R.; Grygier, M.; et al. Clinical Use of Intracoronary Imaging Modalities in Poland. Expert Opinion of the Association of Cardiovascular Interventions of the Polish Cardiac Society. Kardiol. Pol. 2022, 80, 509–519.
- Kini, A.S.; Vengrenyuk, Y.; Pena, J.; Motoyama, S.; Feig, J.E.; Meelu, O.A.; Rajamanickam, A.; Bhat, A.M.; Panwar, S.; Baber, U.; et al. Optical Coherence Tomography Assessment of the Mechanistic Effects of Rotational and Orbital Atherectomy in Severely Calcified Coronary Lesions. Catheter. Cardiovasc. Interv. 2015, 86, 1024–1032.
- Blachutzik, F.; Meier, S.; Weissner, M.; Schlattner, S.; Gori, T.; Ullrich-Daub, H.; Gaede, L.; Achenbach, S.; Möllmann, H.; Chitic, B.; et al. Comparison of Coronary Intravascular Lithotripsy and Rotational Atherectomy in the Modification of Severely Calcified Stenoses. Am. J. Cardiol. 2023, 197, 93–100.
- Nef, H.; Schlattner, S.; Weissner, M.; Gori, T.; Ullrich, H.; Gaede, L.; Achenbach, S.; Möllmann, H.; Blumenstein, J.; Aksoy, A.; et al. TCT-176 Randomized Comparison of Intracoronary Lithotripsy and Rotational Atherectomy for the Treatment of Severely Calcified Vessels—ROTA.Shock Trial. J. Am. Coll. Cardiol. 2022, 80, B71.
- Januszek, R.; Siudak, Z.; Malinowski, K.P.; Wańha, W.; Surowiec, S.; Heba, G.; Pawlik, A.; Kameczura, T.; Wojakowski, W.; Jaguszewski, M.; et al. Factors Determining the Frequency of Optical Coherence Tomography and Intravascular Ultrasound Use in Patients Treated with Percutaneous Coronary Interventions in Recent Years: Analysis Based on a Large National Registry. Kardiol. Pol. 2023, 81, 969–977.
- Kobayashi, N.; Ito, Y.; Yamawaki, M.; Araki, M.; Obokata, M.; Sakamoto, Y.; Mori, S.; Tsutsumi, M.; Honda, Y.; Makino, K.; et al. Optical Coherence Tomography-Guided versus Intravascular Ultrasound-Guided Rotational Atherectomy in Patients with Calcified Coronary Lesions. EuroIntervention 2020, 16, e313–e321.
- Teng, W.; Li, Q.; Ma, Y.; Cao, C.; Liu, J.; Zhao, H.; Lu, M.; Hou, C.; Wang, W. Comparison of Optical Coherence Tomography-Guided and Intravascular Ultrasound-Guided Rotational Atherectomy for Calcified Coronary Lesions. BMC Cardiovasc. Disord. 2021, 21, 290.
- Kurogi, K.; Ishii, M.; Ikebe, S.; Kaichi, R.; Mori, T.; Komaki, S.; Yamamoto, N.; Yamanaga, K.; Arima, Y.; Yamamoto, E.; et al. Optical Coherence Tomography—Versus Intravascular Ultrasound-Guided Stent Expansion in Calcified Lesions. Cardiovasc. Interv. Ther. 2022, 37, 312–323.
- Généreux, P.; Kirtane, A.J.; Kandzari, D.E.; Armstrong, E.J.; Krucoff, M.W.; Redfors, B.; Ben-Yehuda, O.; Lerew, D.R.; Ali, Z.A.; Maehara, A.; et al. Randomized Evaluation of Vessel Preparation with Orbital Atherectomy Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Artery Lesions: Design and Rationale of the ECLIPSE Trial. Am. Heart J. 2022, 249, 1–11.
- Kereiakes, D.J.; Hill, J.M.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions: 1-Year Results from the Disrupt CAD III Study. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100001.
- Mintz, G.S. Intravascular Imaging of Coronary Calcification and Its Clinical Implications. JACC Cardiovasc. Imaging 2015, 8, 461–471.
- Abdel-Wahab, M.; Richardt, G.; Joachim Büttner, H.; Toelg, R.; Geist, V.; Meinertz, T.; Schofer, J.; King, L.; Neumann, F.-J.; Khattab, A.A. High-Speed Rotational Atherectomy before Paclitaxel-Eluting Stent Implantation in Complex Calcified Coronary Lesions. JACC Cardiovasc. Interv. 2013, 6, 10–19.
- Abdel-Wahab, M.; Toelg, R.; Byrne, R.A.; Geist, V.; El-Mawardy, M.; Allali, A.; Rheude, T.; Robinson, D.R.; Abdelghani, M.; Sulimov, D.S.; et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2018, 11, e007415.
- Allali, A.; Abdel-Wahab, M.; Elbasha, K.; Mankerious, N.; Traboulsi, H.; Kastrati, A.; El-Mawardy, M.; Hemetsberger, R.; Sulimov, D.S.; Neumann, F.-J.; et al. Rotational Atherectomy of Calcified Coronary Lesions: Current Practice and Insights from Two Randomized Trials. Clin. Res. Cardiol. 2023, 112, 1143–1163.
- Chambers, J.W.; Feldman, R.L.; Himmelstein, S.I.; Bhatheja, R.; Villa, A.E.; Strickman, N.E.; Shlofmitz, R.A.; Dulas, D.D.; Arab, D.; Khanna, P.K.; et al. Pivotal Trial to Evaluate the Safety and Efficacy of the Orbital Atherectomy System in Treating De Novo, Severely Calcified Coronary Lesions (ORBIT II). JACC Cardiovasc. Interv. 2014, 7, 510–518.
- Shlofmitz, E.; Shlofmitz, R.; Lee, M.S. Orbital Atherectomy: A Comprehensive Review. Interv. Cardiol. Clin. 2019, 8, 161–171.
- Saito, S.; Yamazaki, S.; Takahashi, A.; Namiki, A.; Kawasaki, T.; Otsuji, S.; Nakamura, S.; Shibata, Y. Intravascular Lithotripsy for Vessel Preparation in Calcified Coronary Arteries Prior to Stent Placement―Japanese Disrupt CAD IV Study 1-Year Results. Circ. Rep. 2022, 4, CR-22-0068.