Nasopharyngeal carcinoma (NPC) is a malignancy prevalent in Southeast Asia with the highest metastatic rate amongst other head and neck cancers. The absence of symptoms and unique anatomical positioning often result in late diagnosis, with resistance to chemo/radiotherapy further impacting its prognosis. Tumour-derived exosomes (TEX) have a significant impact on anti-tumour immunity. They can impede the function of immune cells such as T cells and dendritic cells (DCs), preventing them from being activated or inducing their dysfunction. Additionally, TEX can also stimulate the recruitment and production of immunosuppressive cells, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), further weakening the immune response against cancer.
- nasopharyngeal carcinoma
- tumour microenvironment
- exosomes
- extracellular vesicles
- immune checkpoint inhibitors
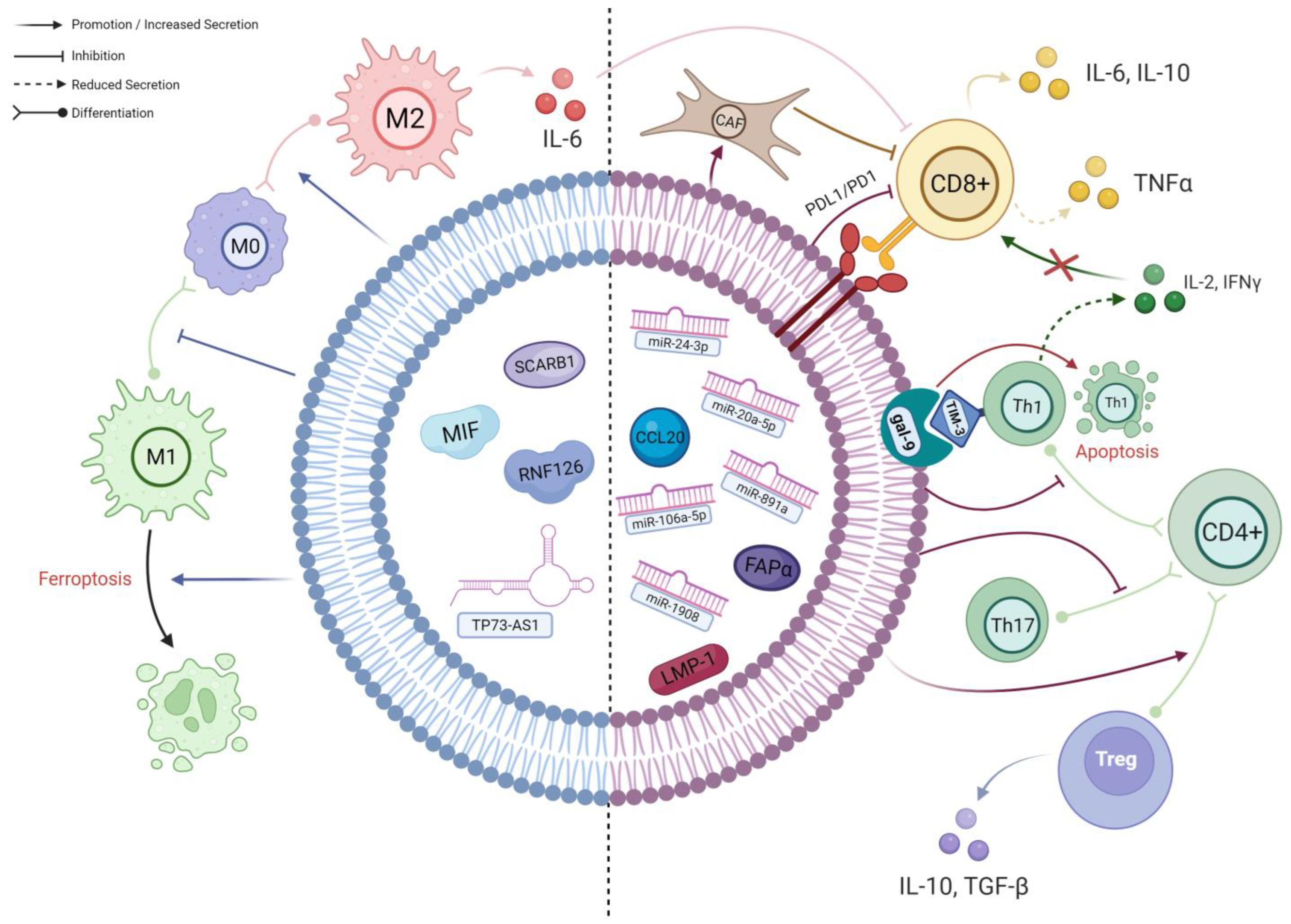
1. TEX Contribute to the Differentiation of Macrophages into Highly Immunosuppressive M2-Polarised Phenotype
2. Exosome-Mediated Inhibition of T Cells in NPC TME
2.1. TEX-Mediated T Cell Inhibition Mechanisms Associated with EBV
2.2. miRNAs in NPC-TEX Alters the Differentiation Patterns and Secretome of T Cells
2.3. NPC-TEX Directly Recruit Tregs to Promote Immune Evasion
2.4. NPC-TEX Surface Proteins Contribute to T Cell Exhaustion
2.5. Potential Pathways of TEX-Mediated T Cell Inhibition Yet to Be Found in NPC
3. Involvement of Exosomes in the Disease Progression and Metastasis of NPC
| Pathogenic Mechanism | Exosome Cargo(s) | Target Gene(s)/Pathway(s) | Effects of Exosome Cargo(s) in Recipient Cells | Ref. |
|---|---|---|---|---|
| 1a. Angiogenesis | ||||
| VEGF Upregulation in ECs |
ICAM-1 | MAPK | Mediates neovascularisation through Src kinase, ERK1/2, p38 MAPK, RhoA/ROCK and eNOS. | [50] |
| miR-17-5p | BAMBI | miR-17-5p downregulate BAMBI, leading to disinhibition of Akt, and thus increased downstream VEGF-A expression. | [51] | |
| miR-144 | FBXW7/HIF-1 α Axis | miR-144 upregulates VEGF-A via the FBXW7/HIF-1α axis. | [52] | |
| Other Mechanisms to Alter ECs’ Properties | STIM1/LMP-1 | Akt/ERK | EBV LMP-1 promotes proliferation, migration, tubulogenesis and permeability in ECs by activating the Akt/ERK pathway. STIM1 was found to promote LMP-1 enrichment in NPC-TEX. | [53] |
| miR-23a | TSGA-10 | miR-23a represses TSGA10 and positively regulates ERK signalling, resulting in proliferation, migration, and formation of tube-like structures in ECs. | [54] | |
| miR-205-5p | DSC2 | miR-205-5p downregulates tumour suppressor DSC2 to promote EGFR/ERK signalling and enhance ECs’ proliferation. | [55] | |
| CCAT2 | Unknown | The lncRNA boosts the proliferation and migration ability of ECs. | [56] | |
| lincROR | p-AKT, p-VEGFR2 | Accelerate the growth of blood vessels, contributing to proliferation, migration and tube formation ability. | [57] | |
| PFKFB3 | ERK, p-AKT | PFKFB3 improve vessel sprouting by regulating cytoskeleton remodelling, migration and tip cell competitiveness. | [58] | |
| HAX-1 | ITGB6 | By upregulating ITGB6, the FAK pathway is activated, leading to higher cell junction permeability and neovascularisation. | [59] | |
| miR-455 | ZO-1 | Under hypoxia, the exosomal miR-455 level is increased, which increases vascular permeability via the inhibition of ZO-1, a protein for endothelial tight junctions. | [60] | |
| ECM Modulation | CD44v5 | Adhesive Proteins | Mediates endothelial cell adhesive proteins and their interactions with ECM components. | [50] |
| EBERs | VCAM-1 | EBERs delivered into ECs are recognised by cytoplasmic TLR-3/RIG-I, activating the downstream ERK1/2/AP1 axis, thus stimulating VCAM-1 adhesive protein expression. | [61] | |
| miR-205-5p | DSC2 | Inhibition of DSC2 by miR-205-5p enhances EGFR/ERK signalling and MMP-2, -9 expression such that extracellular matrix proteins are degraded and remodelled. | [55] | |
| 1b. Metastasis | ||||
| Promoting Tumour Intravasation | HMGA2 | Snail | HMGA2 is overexpressed in EBV-infected NPC and their TEX. It upregulates Snail in ECs, promoting mesenchymal transition and degrading tight junctions, increasing vascular permeability. | [62] |
| Inducing EMT in NPC Cells | MMP-13 | AKT1, ERK1/2 |
NPC-TEX is often enriched in MMP-13. Overexpression of HIF-1α in NPC cells under hypoxia is a probable cause. Exosomal MMP-13 could induce EMT in normoxic tumour cells possibly through AKT1 and ERK1/2 signalling. | [63] |
| miR-106a-5p | FBXW7- TRIM24- SRGN Axis | Exosomal miR-106a 5p suppresses FBXW7 to downregulate FBXW7-mediated ubiquitin degradation of TRIM24. Thus, more TRIM24 binds to SRGN promoter region, and SRGN then promotes metastasis through EMT. | [64] | |
| miR-18a-5p | BTG3 | EMT markers are induced by TEX miR-18a-5p in NPC cells by suppressing BTG3 and activating the Wnt/β-catenin pathway. | [65] | |
| miR-301a-3p | BTG1 | Aberrant expression of miR-301a-3p promotes the proliferation, migration, invasion and EMT of NPC cells by suppressing BTG1 mRNA, a tumour suppressor gene. | [66] | |
This entry is adapted from the peer-reviewed paper 10.3390/cancers16050919
References
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9.
- Chen, P.; Bonaldo, P. Role of macrophage polarization in tumor angiogenesis and vessel normalization: Implications for new anticancer therapies. Int. Rev. Cell Mol. Biol. 2013, 301, 1–35.
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389.
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555.
- Elgert, K.D.; Alleva, D.G.; Mullins, D.W. Tumor-induced immune dysfunction: The macrophage connection. J. Leukoc. Biol. 1998, 64, 275–290.
- Barbosa de Souza Rizzo, M.; Brasilino de Carvalho, M.; Kim, E.J.; Rendon, B.E.; Noe, J.T.; Darlene Wise, A.; Mitchell, R.A. Oral squamous carcinoma cells promote macrophage polarization in an MIF-dependent manner. QJM: Int. J. Med. 2018, 111, 769–778.
- Chen, W.; Zuo, F.; Zhang, K.; Xia, T.; Lei, W.; Zhang, Z.; Bao, L.; You, Y. Exosomal MIF Derived From Nasopharyngeal Carcinoma Promotes Metastasis by Repressing Ferroptosis of Macrophages. Front. Cell Dev. Biol. 2021, 9, 791187.
- Yu, C.; Xue, B.; Li, J.; Zhang, Q. Tumor cell-derived exosome RNF126 affects the immune microenvironment and promotes nasopharyngeal carcinoma progression by regulating PTEN ubiquitination. Apoptosis Int. J. Program. Cell Death 2022, 27, 590–605.
- Xu, H.; Ju, L.; Xiong, Y.; Yu, M.; Zhou, F.; Qian, K.; Wang, G.; Xiao, Y.; Wang, X. E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability. Cell Death Dis. 2021, 12, 239.
- Shan, M.; Qin, J.; Jin, F.; Han, X.; Guan, H.; Li, X.; Zhang, J.; Zhang, H.; Wang, Y. Autophagy suppresses isoprenaline-induced M2 macrophage polarization via the ROS/ERK and mTOR signaling pathway. Free Radic. Biol. Med. 2017, 110, 432–443.
- Feng, X.; Ji, Y.; Zhang, C.; Jin, T.; Li, J.; Guo, J. CCL6 promotes M2 polarization and inhibits macrophage autophagy by activating PI3-kinase/Akt signalling pathway during skin wound healing. Exp. Dermatol. 2023, 32, 403–412.
- Gao, Z.; Li, X.G.; Feng, S.-R.; Chen, J.F.; Song, K.; Shi, Y.H.; Tang, Z.; Liu, W.R.; Zhang, X.; Huang, A.; et al. Autophagy suppression facilitates macrophage M2 polarization via increased instability of NF-κB pathway in hepatocellular carcinoma. Int. Immunopharmacol. 2023, 123, 110685.
- Yao, H.; Tian, L.; Yan, B.; Yang, L.; Li, Y. LncRNA TP73-AS1 promotes nasopharyngeal carcinoma progression through targeting miR-342-3p and M2 polarization via exosomes. Cancer Cell Int. 2022, 22, 16.
- Chen, W.; Bao, L.; Ren, Q.; Zhang, Z.; Yi, L.; Lei, W.; Yang, Z.; Lu, Y.; You, B.; You, Y.; et al. SCARB1 in extracellular vesicles promotes NPC metastasis by co-regulating M1 and M2 macrophage function. Cell Death Discov. 2023, 9, 323.
- Wang, X.; Xiang, Z.; Tsao, G.S.; Tu, W. Exosomes derived from nasopharyngeal carcinoma cells induce IL-6 production from macrophages to promote tumorigenesis. Cell Mol. Immunol. 2021, 18, 501–503.
- Nakamura, K.; Smyth, M.J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol. Immunol. 2020, 17, 1–12.
- Lin, W.-W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183.
- Zhang, G.; Tsang, C.M.; Deng, W.; Yip, Y.L.; Lui, V.W.-Y.; Wong, S.C.C.; Cheung, A.L.-M.; Hau, P.M.; Zeng, M.; Lung, M.L.; et al. Enhanced IL-6/IL-6R Signaling Promotes Growth and Malignant Properties in EBV-Infected Premalignant and Cancerous Nasopharyngeal Epithelial Cells. PLoS ONE 2013, 8, e62284.
- Kato, T.; Noma, K.; Ohara, T.; Kashima, H.; Katsura, Y.; Sato, H.; Komoto, S.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-Associated Fibroblasts Affect Intratumoral CD8(+) and FoxP3(+) T Cells Via IL6 in the Tumor Microenvironment. Clin. Cancer Res. 2018, 24, 4820–4833.
- Ferradini, L.; Miescher, S.; Stoeck, M.; Busson, P.; Barras, C.; Cerf-Bensussan, N.; Lipinski, M.; Von Fliedner, V.; Tursz, T. Cytotoxic potential despite impaired activation pathways in T lymphocytes infiltrating nasopharyngeal carcinoma. Int. J. Cancer 1991, 47, 362–370.
- Lakhdar, M.; Aribia, M.H.B.; Maalej, M.; Ladgham, A. Selective homing of phenotypically lytic cells within nasopharyngeal carcinoma biopsies: Numerous CD8- and CD16-positive cells in the tumor. Int. J. Cancer 2007, 48, 57–61.
- Liu, Y.; He, S.; Wang, X.-L.; Peng, W.; Chen, Q.-Y.; Chi, D.-M.; Chen, J.-R.; Han, B.-W.; Lin, G.-W.; Li, Y.-Q.; et al. Tumour heterogeneity and intercellular networks of nasopharyngeal carcinoma at single cell resolution. Nat. Commun. 2021, 12, 741.
- Lau, K.M.; Cheng, S.H.; Lo, K.W.; Lee, S.A.K.W.; Woo, J.K.S.; Van Hasselt, C.A.; Lee, S.P.; Rickinson, A.B.; Ng, M.H.L. Increase in circulating Foxp3+CD4+CD25high regulatory T cells in nasopharyngeal carcinoma patients. Br. J. Cancer 2007, 96, 617–622.
- Keryer-Bibens, C.; Pioche-Durieu, C.; Villemant, C.; Souquère, S.; Nishi, N.; Hirashima, M.; Middeldorp, J.; Busson, P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer 2006, 6, 283.
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252.
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Le Moulec, S.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009, 113, 1957–1966.
- Lee, P.J.; Sui, Y.H.; Liu, T.T.; Tsang, N.M.; Huang, C.H.; Lin, T.Y.; Chang, K.P.; Liu, S.C. Epstein-Barr viral product-containing exosomes promote fibrosis and nasopharyngeal carcinoma progression through activation of YAP1/FAPα signaling in fibroblasts. J. Exp. Clin. Cancer Res. 2022, 41, 254.
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598.
- Hilmi, M.; Nicolle, R.; Bousquet, C.; Neuzillet, C. Cancer-Associated Fibroblasts: Accomplices in the Tumor Immune Evasion. Cancers 2020, 12, 2969.
- Ye, S.B.; Li, Z.L.; Luo, D.H.; Huang, B.J.; Chen, Y.S.; Zhang, X.S.; Cui, J.; Zeng, Y.X.; Li, J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 2014, 5, 5439–5452.
- Ye, S.B.; Zhang, H.; Cai, T.T.; Liu, Y.N.; Ni, J.J.; He, J.; Peng, J.Y.; Chen, Q.Y.; Mo, H.Y.; Jun, C.; et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340.
- Cao, Q.; Li, Y.Y.; He, W.F.; Zhang, Z.Z.; Zhou, Q.; Liu, X.; Shen, Y.; Huang, T.T. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol. Genom. 2013, 45, 1206–1214.
- Olalekan, S.A.; Cao, Y.; Hamel, K.M.; Finnegan, A. B cells expressing IFN-γ suppress Treg-cell differentiation and promote autoimmune experimental arthritis. Eur. J. Immunol. 2015, 45, 988–998.
- Caretto, D.; Katzman, S.D.; Villarino, A.V.; Gallo, E.; Abbas, A.K. Cutting Edge: The Th1 Response Inhibits the Generation of Peripheral Regulatory T Cells. J. Immunol. 2010, 184, 30–34.
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15.
- Calzascia, T.; Pellegrini, M.; Hall, H.; Sabbagh, L.; Ono, N.; Elford, A.R.; Mak, T.W.; Ohashi, P.S. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J. Clin. Invest. 2007, 117, 3833–3845.
- Mrizak, D.; Martin, N.; Barjon, C.; Jimenez-Pailhes, A.S.; Mustapha, R.; Niki, T.; Guigay, J.; Pancré, V.; de Launoit, Y.; Busson, P.; et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J. Natl. Cancer Inst. 2015, 107, 363.
- Yang, L.; Liu, G.; Li, Y.; Pan, Y. The emergence of tumor-infiltrating lymphocytes in nasopharyngeal carcinoma: Predictive value and immunotherapy implications. Genes Dis. 2022, 9, 1208–1219.
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386.
- Yang, J.; Chen, J.; Liang, H.; Yu, Y. Nasopharyngeal cancer cell-derived exosomal PD-L1 inhibits CD8+ T-cell activity and promotes immune escape. Cancer Sci. 2022, 113, 3044–3054.
- Yilmaz, E.; Ismaila, N.; Bauman, J.E.; Dabney, R.; Gan, G.; Jordan, R.; Kaufman, M.; Kirtane, K.; McBride, S.M.; Old, M.O.; et al. Immunotherapy and Biomarker Testing in Recurrent and Metastatic Head and Neck Cancers: ASCO Guideline. J. Clin. Oncol. 2023, 41, 1132–1146.
- Guan, L.; Wu, B.; Li, T.; Beer, L.A.; Sharma, G.; Li, M.; Lee, C.N.; Liu, S.; Yang, C.; Huang, L.; et al. HRS phosphorylation drives immunosuppressive exosome secretion and restricts CD8+ T-cell infiltration into tumors. Nat. Commun. 2022, 13, 4078.
- Tesone, A.J.; Svoronos, N.; Allegrezza, M.J.; Conejo-Garcia, J.R. Pathological mobilization and activities of dendritic cells in tumor-bearing hosts: Challenges and opportunities for immunotherapy of cancer. Front. Immunol. 2013, 4, 435.
- Maus, R.L.G.; Jakub, J.W.; Nevala, W.K.; Christensen, T.A.; Noble-Orcutt, K.; Sachs, Z.; Hieken, T.J.; Markovic, S.N. Human Melanoma-Derived Extracellular Vesicles Regulate Dendritic Cell Maturation. Front. Immunol. 2017, 8, 358.
- Wang, M.; Cai, Y.; Peng, Y.; Xu, B.; Hui, W.; Jiang, Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020, 11, 896.
- Salimu, J.; Webber, J.; Gurney, M.; Al-Taei, S.; Clayton, A.; Tabi, Z. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracell Vesicles 2017, 6, 1368823.
- Lorusso, G.; Rüegg, C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem. Cell Biol. 2008, 130, 1091–1103.
- Wandrey, M.; Jablonska, J.; Stauber, R.H.; Gül, D. Exosomes in Cancer Progression and Therapy Resistance: Molecular Insights and Therapeutic Opportunities. Life 2023, 13, 2033.
- Yokota, J. Tumor progression and metastasis. Carcinogenesis 2000, 21, 497–503.
- Chan, Y.K.; Zhang, H.; Liu, P.; Tsao, S.W.; Lung, M.L.; Mak, N.K.; Ngok-Shun Wong, R.; Ying-Kit Yue, P. Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int. J. Cancer 2015, 137, 1830–1841.
- Duan, B.; Shi, S.; Yue, H.; You, B.; Shan, Y.; Zhu, Z.; Bao, L.; You, Y. Exosomal miR-17-5p promotes angiogenesis in nasopharyngeal carcinoma via targeting BAMBI. J. Cancer 2019, 10, 6681–6692.
- Tian, X.; Liu, Y.; Wang, Z.; Wu, S. miR-144 delivered by nasopharyngeal carcinoma-derived EVs stimulates angiogenesis through the FBXW7/HIF-1α/VEGF-A axis. Mol. Ther. Nucleic Acids 2021, 24, 1000–1011.
- Deng, Y.; Liu, X.; Huang, Y.; Ye, J.; He, Q.; Luo, Y.; Chen, Y.; Li, Q.; Lin, Y.; Liang, R.; et al. STIM1-regulated exosomal EBV-LMP1 empowers endothelial cells with an aggressive phenotype by activating the Akt/ERK pathway in nasopharyngeal carcinoma. Cell Oncol. 2023, 46, 987–1000.
- Bao, L.; You, B.; Shi, S.; Shan, Y.; Zhang, Q.; Yue, H.; Zhang, J.; Zhang, W.; Shi, Y.; Liu, Y.; et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018, 37, 2873–2889.
- Yang, W.; Tan, S.; Yang, L.; Chen, X.; Yang, R.; Oyang, L.; Lin, J.; Xia, L.; Wu, N.; Han, Y.; et al. Exosomal miR-205-5p enhances angiogenesis and nasopharyngeal carcinoma metastasis by targeting desmocollin-2. Mol. Ther. Oncolytics 2022, 24, 612–623.
- Zhou, S.K.; Gao, F.; Zhong, Z.S.; Yao, H. Long non-coding RNA colon cancer associated transcript-2 from nasopharyngeal carcinoma-derived exosomes promotes angiogenesis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 55, 944–951.
- Zhang, S.; Cai, J.; Ji, Y.; Zhou, S.; Miao, M.; Zhu, R.; Li, K.; Xue, Z.; Hu, S. Tumor-derived exosomal lincRNA ROR promotes angiogenesis in nasopharyngeal carcinoma. Mol. Cell Probes 2022, 66, 101868.
- Gu, M.; Li, L.; Zhang, Z.; Chen, J.; Zhang, W.; Zhang, J.; Han, L.; Tang, M.; You, B.; Zhang, Q.; et al. PFKFB3 promotes proliferation, migration and angiogenesis in nasopharyngeal carcinoma. J. Cancer 2017, 8, 3887–3896.
- You, B.; Cao, X.; Shao, X.; Ni, H.; Shi, S.; Shan, Y.; Gu, Z.; You, Y. Clinical and biological significance of HAX-1 overexpression in nasopharyngeal carcinoma. Oncotarget 2016, 7, 12505–12524.
- Xie, L.; Zhang, K.; You, B.; Yin, H.; Zhang, P.; Shan, Y.; Gu, Z.; Zhang, Q. Hypoxic nasopharyngeal carcinoma-derived exosomal miR-455 increases vascular permeability by targeting ZO-1 to promote metastasis. Mol. Carcinog. 2023, 62, 803–819.
- Cheng, S.; Li, Z.; He, J.; Fu, S.; Duan, Y.; Zhou, Q.; Yan, Y.; Liu, X.; Liu, L.; Feng, C.; et al. Epstein-Barr virus noncoding RNAs from the extracellular vesicles of nasopharyngeal carcinoma (NPC) cells promote angiogenesis via TLR3/RIG-I-mediated VCAM-1 expression. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1201–1213.
- Li, D.K.; Chen, X.R.; Wang, L.N.; Wang, J.H.; Li, J.K.; Zhou, Z.Y.; Li, X.; Cai, L.B.; Zhong, S.S.; Zhang, J.J.; et al. Exosomal HMGA2 protein from EBV-positive NPC cells destroys vascular endothelial barriers and induces endothelial-to-mesenchymal transition to promote metastasis. Cancer Gene. Ther. 2022, 29, 1439–1451.
- Shan, Y.; You, B.; Shi, S.; Shi, W.; Zhang, Z.; Zhang, Q.; Gu, M.; Chen, J.; Bao, L.; Liu, D.; et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis. 2018, 9, 382.
- Li, C.W.; Zheng, J.; Deng, G.Q.; Zhang, Y.G.; Du, Y.; Jiang, H.Y. Exosomal miR-106a-5p accelerates the progression of nasopharyngeal carcinoma through FBXW7-mediated TRIM24 degradation. Cancer Sci. 2022, 113, 1652–1668.
- Zhong, Q.; Nie, Q.; Wu, R.; Huang, Y. Exosomal miR-18a-5p promotes EMT and metastasis of NPC cells via targeting BTG3 and activating the Wnt/β-catenin signaling pathway. Cell Cycle 2023, 22, 1544–1562.
- Cheng, Q.; Li, Q.; Xu, L.; Jiang, H. Exosomal microRNA-301a-3p promotes the proliferation and invasion of nasopharyngeal carcinoma cells by targeting BTG1 mRNA. Mol. Med. Rep. 2021, 23, 328.
