1. Introduction
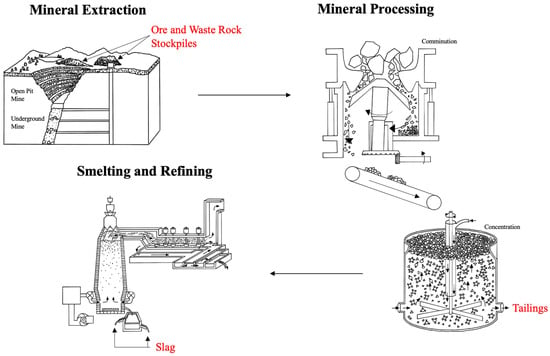
Environmental engineering is a broad discipline encompassing a large spectrum of prevention, reduction, recovery, and treatment efforts. While treatment measures are at the bottom of the environmental management hierarchy, they are essential to the longevity and prosperity of the geo-environment. Remediation refers to a reversal of or reduction in environmental damage. This includes waste management from anthropogenic activities, including mining operations. Mining waste from both dated (orphaned and abandoned mines) and developing projects is a significant issue worldwide. Waste is generated from the extraction process (surface and underground mining), mineral processing (comminution, classification, and concentration), and smelting and refining (Figure 1). Waste from each of these operations gets piled in the nearby environment, where it is subject to oxidation and can leach into the surrounding soil and groundwater. As a result, it can cause metal(loid) contamination that is highly acidic or highly alkaline, causing risk to the nearby environment and ecosystems.
Figure 1. Solid mining waste from mining industry operations, including ore and waste rock stockpiles after mineral extraction, tailings after mineral processing, and slag after smelting. Each of these solid wastes can leach contaminants into the soil and groundwater (adapted from [
1,
2,
3,
4]).
Mining is an essential part of society necessary to maintain the quality of life for a vastly growing population and to ensure development and progress. While mining has progressed over the years to consider and incorporate environmental approaches, the severe contamination produced by current and historical mine activities has created considerable ecological damage. Mine-related contamination is often derived from the poor management of stockpiled ore and waste rock, tailings, and slag dumps. These waste piles are impacted by changing environmental conditions. Redox changes, for example, can increase the toxicity and/or mobility of contamination, causing leachate to migrate to soil, groundwater, and surface water sources. Since there is a continued demand for minerals and over-extraction ensures more complex mining operations, it is likely that the volume of waste will increase with decreased ore cut-off grades [
5]. This will increase the likelihood of leachate contamination. Remediation is therefore essential to the mitigation of point source contamination from mining activities. It is important that these contaminated sites be monitored and managed to reduce the fate and transport of metal(loid)s, which impact the environment and can deteriorate human and animal health.
Remediation, specifically bioremediation, is crucial for the green and sustainable treatment of mining waste. Many environmental engineering technologies have high costs, long response times, and/or non-compliant leachate rates or metal concentrations post reclamation. Biological remediation techniques have the potential to mitigate the environmental impact experienced during cleanup and pose an advantageous solution for the field of remediation. MICP is a green and sustainable geotechnical engineering technique. The method facilitates chemical precipitation with various microorganism species to precipitate solid CaCO3 polymorphs, creating a biocement matrix. Other terms used synonymously for MICP include biocementation, bacterial carbonatogenesis, and biocalcification.
2. Biochemical Processes
2.1. MICP
MICP is a biological enhancement to chemical precipitation processes. The reactions are biochemical, utilizing microorganisms as a catalyst to facilitate precipitation. As with chemical precipitation, the reactions are thermodynamically and kinetically driven. Precipitation is governed by a thermodynamic state of instability, whereby the solute concentration exceeds the liquid–solid equilibrium (supersaturation state) causing precipitates to form [
6,
7]. Kinetically, these precipitates develop through nucleation, growth, and agglomeration, referring to the birth and enlargement of particles [
7].
Biologically, this process can occur naturally using indigenous microorganisms (biostimulation), or with the addition of exogenous microorganisms (bioaugmentation). Indigenous microorganisms can adapt to their environment and develop a resistance to the toxicity of the contamination, specifically creating a tolerance to high metal(loid) concentrations [
8,
9]. However, if microorganisms are not naturally present or a specific strain is not available, exogenous microorganisms can be added to facilitate the process. In both scenarios, the microorganisms require nutrients and energy to stimulate growth. The application of a nutrient broth (NB) is required to enhance microbial survival, especially within nutrient-deficient mining waste. The microorganisms precipitate solids through biologically mediated mechanisms (passive precipitation caused by organic matter from microbial activity), biologically controlled mechanisms (direct precipitation from cellular activity), or biologically induced mechanisms (direct precipitation resulting from environmental changes caused by biological activity) [
10,
11].
MICP is a biologically induced mechanism. It occurs from direct biological activity that alters the extracellular environment. These microbial pathways include enzyme activity, oxidation–reduction reactions, or photosynthesis processes. Microorganisms can release organic acids, electron donors, and enzymes into the extracellular environment through passive diffusion, secretion, and active pumping [
11,
12,
13,
14]. Again, this impacts the extracellular environment, which can induce precipitation.
The microbial enzymes urease and carbonic anhydrase (CA) facilitate CaCO
3 precipitation from urea hydrolysis and carbon dioxide (CO
2) hydration, respectively. These enzymes can work together to precipitate CaCO
3, since urease converts urea ((NH
2)
2CO) into ammonia (NH
3), then ammonium (NH
4+), and CA converts carbonic acid (H
2CO
3) to bicarbonate (HCO
3−), then carbonate (CO
32−) [
11]. However, CA can also precipitate CaCO
3 independently. It can act as a catalyst for the transformation of atmospheric CO
2 into MCO
3 compounds, which can enhance precipitation [
15]. The redox-driven forms of MICP (denitrification, sulfate reduction, iron reduction, methane oxidation, and ammonification [
16,
17]) utilize oxidative–reductive reactions to enable precipitation. The redox transformations alter the solubility [
18,
19], which alters the saturation and supersaturation states, leading to precipitation [
20]. Finally, photosynthetic bacteria (cyanobacteria and microalgae [
11]) facilitate precipitation via the synthesis of atmospheric CO
2 into organic matter and simultaneous HCO
3−/OH
− exchange across the cell membrane, followed by CaCO
3 precipitation within the cell (excess Ca
2+ is stored in the cell membrane), or extracellularly via an antiporter [
17]. In all situations, precipitation favors high pH conditions.
2.2. Mining Waste Characterization and Treatment
The type of MICP mechanism utilized will be dependent on the indigenous microorganisms at the site (i.e., biostimulation) and the type of waste. With respect to pH of the leachate generated, mining waste can be categorized into three groups: acidic, neutral, and alkaline waste. During mineral extraction and processing, overburden material is stockpiled, and tailings are discharged into tailing storage facilities (TSFs). Tailings typically consist of ore residues which can leach into the soil and groundwater, causing contamination. The leachate behavior is based on the chemistry of the infiltrating water and receiving water, the composition and age of the materials including co-deposited wastes, and the geophysical site (topography, soil porosity and permeability, flow rates, redox potential, etc.) [
25]. This water balance will influence the fate and transport of soluble contaminants, such as metal(loid)s, in mining waste.
Acid rock drainage (ARD), or acid mine drainage (AMD), refers to contaminated, acidic drainage water, typically originating from mines or mining activities. The generation process of AMD involves the oxidation of sulfur or sulfide (S
2−) minerals, whereby Equation (1) shows the oxidation of pyrite (FeS
2) into ferric hydroxide (Fe(OH)
3) and sulfuric acid (SO
42− and H
+) [
26]. AMD is characterized by low pH and high concentrations of sulfates and metal(loid)s [
27]. The rate of AMD generation is dependent on sulfide morphology, oxygen content, wetting/drying cycles, microbial activity, and geologic history [
28]. Sulfate reduction via sulfate-reducing bacteria (SRB) is a prevalent method to treat AMD using MICP. A carbon source (electron donor) facilitates the dissimilatory sulfate reduction from SO
42− to H
2S [
24]. The reaction releases CO
2 and hydroxide ions (OH
−) which are utilized in the formation of solid CaCO
3.
Alkaline waste is derived from the hydration of alkaline earth oxides. With mine operations, it is often associated with nickel, chrysotile, kimberlite, and red mud mining [
29]. With mineral processing operations, it is often associated with gold, alumina, chromite, and uranium [
5]. Equation (2) demonstrates a generic chemical reaction utilizing calcium oxide (CaO), although magnesium oxide (MgO), sodium oxide (Na
2O), and ferrous oxide (FeO) are also common [
25,
29,
30]. Alkaline waste is characterized by a high content of alkaline earth metals [
29], high pH, high salinity, high sodicity, and fine particle size [
5]. Alkaline waste from mining leachate can cause high pH, high chemical oxygen demand, oxygen depletion, high sulfate loadings, salinity, and high concentrations of metal precipitates [
25].
Alkaline waste can be treated using MICP via microbial activity from the CA enzyme, which transforms CO
2 to H
2CO
3. The H
2CO
3 will react with the calcium hydroxide (Ca(OH)
2) from Equation (2) to produce CaCO
3, as shown in Equation (3) [
30]. In addition, carbonate precipitation can mimic the natural weathering process of silicate minerals [
25,
29]. This is shown in Equation (4), whereby the dissolution of alkaline earth metals from the silicate matrix is required for carbonate precipitation. This can occur as a direct process (occurs in one step: dissolution and precipitation) or an indirect process (occurs as two steps: dissolution via lixiviate, then precipitation) [
25,
31]. The carbonate precipitation of alkaline waste is impacted by the solid-to-liquid ratio, particle size, and temperature [
25].
The type and characteristics of mining waste will influence the MICP design. MICP can be utilized as a remediation strategy to treat metal(loid) contamination that is highly acidic or alkaline. The geophysical mechanisms involved in the remediation of solid mining waste (waste stockpiles, tailings, and slag dumps) are detailed as follows.
3. Bioremediation Processes—Geophysical and Biochemical Interactions
3.1. Bioremediation
Precipitation (and/or co-precipitation (CaCO
3/MCO
3)) creates a biocement matrix formed by the interconnection of CaCO
3 precipitates. At the microscale (via microbial activity), precipitates form uniformly around soil particles or between particle–particle contacts. Effective bridges are formed at the pore throats due to capillary force. In both scenarios, the precipitates decrease pore space and reduce hydraulic conductivity [
32]. They also reduce soil void volume and increase soil cohesion, which decreases permeability and causes a plugging effect [
33]. Further, particle–particle precipitation and the creation of effective bridges is speculated to improve soil, specifically shear strength [
32].
MICP remediates metal(loid) contamination through removal, immobilization, impermeable barriers, and liquefaction reduction. The removal of metal(loid) contamination is due to the direct precipitation of CaCO
3 and MCO
3 minerals, while immobilization is attributed to the decrease in leachate caused by MICP [
33]. The reduction in leachate is often assessed through the decrease in the soluble–exchangeable fraction, which indicates contaminant bioavailability and mobility [
18,
22,
34,
35,
36]. Leachate is also reduced by the surface deposition and clogging of pore spaces via CaCO
3 crystals [
21,
37]. The development of the biocement matrix will create an impermeable barrier by establishing a plugging effect [
38,
39]. For specificity, remediation via MICP can be direct (fixed in CaCO
3 or MCO
3 precipitates [
35,
39,
40,
41]) or indirect (via metal(loid)-CaCO
3 complexes [
21,
22,
42], inclusion in the crystal structure [
35,
40,
42], and/or sorption [
35,
39]). The inclusion of metal(loid)s into the crystal structure occurs from divalent cations similar to Ca
2+ ions (i.e., ion radius and ion charge), which are integrated into the crystal matrix by substitution/ion exchange, or are integrated via fissures and/or interstices [
42]. Further, sorption can refer to the adsorptive properties of CaCO
3, which has been used as an adsorbent for metal(loid) removal [
43,
44].
Again, the surface deposition of CaCO
3 and precipitation within the pore spaces will create a plugging effect or impermeable barrier. The precipitation of CaCO
3 crystals at the surface of waste piles can negate physical degradation (via wetting and drying (W/D), freezing and thawing (F/T), hot and cold (H/C), wind, percolating fluids, erosion, physical loading stresses, etc.) [
22,
47,
48,
49]. This crust can decrease water absorption and permeability through the specimen [
48,
50], reducing metal(loid)-contaminated leachate [
21,
37]. The precipitation of CaCO
3 crystals within the pore spaces will also decrease permeability, while simultaneously increasing strength [
22,
50]. Comprehensive strength is increased through the consolidation of biologically induced CaCO
3 precipitates [
50], whereby precipitates form in the pore spaces within sample fractures and fissures. This can reduce liquefaction, specifically tailing liquefaction, since the biocement matrix (i.e., particle–particle precipitates) reduces pore water pressure [
33].
Microorganisms, once introduced to the system, will transport and adsorb to solid particles [
51], acting as a location for nucleation and growth [
14]. The bacterial cell wall has a negative surface charge due to carboxyl, phosphoryl, amino, and sulfo groups [
13,
52]. This can attract heavy metals and metalloids, causing adsorption, redox changes, and precipitation [
53]. In addition, extracellular polymeric substances (EPSs) and biofilm formation can reduce pore size, hydraulic conductivity, and permeability [
54], again decreasing fluid movement through the contaminated area. Precipitation at the biofilm boundary can coat pore space and individual grains [
21]. The EPSs from microorganisms have strong metal(loid)-binding capacity [
46], and metal(loid) ions can adsorb on functional groups of EPSs [
20]. This means metal(loid) contaminants in soil and groundwater may be immobilized via microbial EPS secretion. While this is not the direct effect of MICP (a biologically induced mechanism), it is considered a biologically mediated mechanism [
10,
11]. Passive CaCO
3 precipitation occurs when a pH increase causes functional groups to deprotonate, creating a negative charge of EPS, and leading Ca
2+ ions to bind and precipitate [
11].
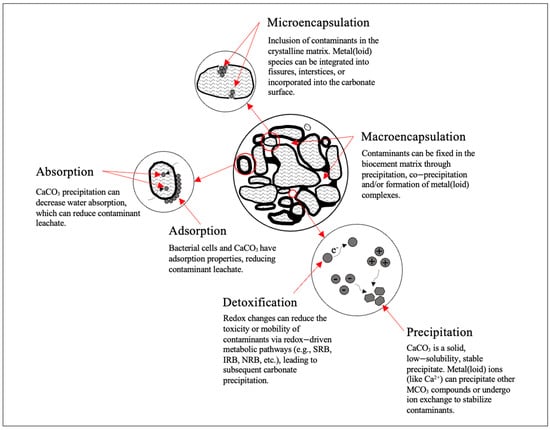
As a biological remediation technique, MICP is bio-physiochemical process whereby metal(loid) species are immobilized or fixated at the site (in situ). The mechanisms attributed to its efficacy as a remediation strategy are detailed in
Figure 2. These mechanisms (macroencapsulation, microencapsulation, absorption, adsorption, precipitation, and detoxification) are inspired by the well-established solidification/stabilization technique [
47], whereby MICP broadly mimics the immobilization mechanisms [
55]. However, this process is not without disadvantages. Metal(loid)s can be released or redissolved with physical degradation, physiochemical stress, or changes to their environmental conditions (i.e., pH, redox conditions).
Figure 2. Mechanisms of bioremediation via MICP and details of the immobilization of fixation of metal(loid) contaminant species.
This entry is adapted from the peer-reviewed paper 10.3390/toxics12020107