Gliomas, the most prevalent primary malignant brain tumors, present a challenging prognosis even after undergoing surgery, radiation, and chemotherapy. Exosomes, nano-sized extracellular vesicles secreted by various cells, play a pivotal role in glioma progression and contribute to resistance against chemotherapy and radiotherapy by facilitating the transportation of biological molecules and promoting intercellular communication within the tumor microenvironment. Moreover, exosomes exhibit the remarkable ability to traverse the blood–brain barrier, positioning them as potent carriers for therapeutic delivery. These attributes hold promise for enhancing glioma diagnosis, prognosis, and treatment.
1. Role of Exosomes in Glioma Progression
Exosomes play a vital role in facilitating cell-to-cell communication by transporting bioactive molecules from donor to recipient cells [
22]. Studies have demonstrated that cancer cells, including gliomas, release exosomes abundantly to communicate with neighboring cells both locally and distantly [
23]. Glioma-derived exosomes (GDEs) exert influence on various aspects of tumor development and progression, including the establishment of the pre-metastatic niche, immune evasion, angiogenesis, anti-apoptotic signaling, and resistance to treatment. Conversely, exosomes from healthy cells, such as T cells, B cells, and dendritic cells, have been shown to significantly inhibit tumor growth [
59]. This multifaceted impact is attributed to various proteins, mRNAs, and noncoding RNAs carried by exosomes (
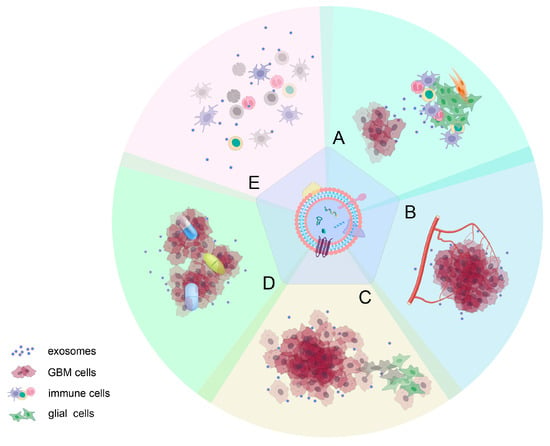
Figure 2).
Figure 2. Roles of GDEs in glioma progression: (A) inducing changes in the tumor microenvironment; (B) mediating angiogenesis; (C) influencing proliferation and invasiveness of gliomas; (D) contributing to drug resistance; and (E) suppressing immune responses. It is important to note that the effects of active molecules on tumor progression are often not isolated but rather have a wide-ranging impact on various aspects of glioma development.
1.1. Effect on the Microenvironment around Gliomas
The tumor microenvironment (TME) in gliomas encompasses a diverse array of both cancer and non-cancer cells, including endothelial cells, immune cells, glioma stem-like cells, and non-cellular components like the extracellular matrix [
60]. The TME has emerged as a powerful driver of glioma progression and a critical regulator of tumor growth [
61]. Exosomes have been identified as crucial mediators of communication between the tumor and the TME. For instance, a study revealed that the miR-340-5p-macrophage feedback loop altered both GBM development and the TME [
62]. Another study demonstrated that tumor-suppressive miR-3591-3p could be released via exosomes to target tumor-associated macrophages in glioma cells, thus creating a suppressive microenvironment that facilitated glioma invasion and migration [
63].
1.2. Angiogenesis Mediated by Exosomes
Exosomes released by gliomas play a pivotal role in driving angiogenesis, a critical process in glioma progression. For example, a study investigating the impact of glioma cells on angiogenesis found that glioma cells could enhance this process by delivering Linc-CCAT2 to endothelial cells via exosomes [
64]. Additionally, research by Li Yan et al. highlighted the significance of exosome-derived circGLIS3 in high-grade glioma, where it contributes to glioma invasion and angiogenesis by regulating Ezrin T567 phosphorylation [
65]. Notably, ECRG4-EV generated from brain endothelial cells has been shown to inhibit glioma growth by regulating angiogenesis and inflammation [
66].
1.3. Role of Exosomes in the Proliferation and Invasiveness of Gliomas
Glioma-derived exosomes (GDEs) contain both coding and noncoding RNA elements, rendering them pivotal in regulating tumor growth, survival, and invasion, crucial aspects of glioma cell persistence and recurrence [
67]. For instance, the presence of exosomal miR-148a, known to target CAMD1 and boost signal transducer and activator of transcription 3 (STAT3) activity, has been linked to the advancement of glioblastoma multiforme (GBM) [
68]. Studies have confirmed that both overexpression of miR-486-5p and silencing of circZNF652 hindered cell proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) in GBM cells. Notably, GBM-cell-derived exosomes were characterized by highly expressed CircZNF652 [
69]. Furthermore, research has demonstrated that miR-3184-3p, circARID1A, and miR-9, abundant in GDEs, fostered the proliferation, invasion, and migration of glioma cells [
70,
71,
72].
1.4. Role of Exosomes in the Resistance to Glioma Treatment
Exosomes play a pivotal role in the resistance exhibited by gliomas against treatment, as they can transport drugs out of tumor cells [
73]. They may stimulate the development of fibroblasts that lead to fibroblastic responses, acting as a barrier against anti-tumor medications. Additionally, through biomolecules such as miRNA, exosomes can transform drug-sensitive tumor cells into drug-resistant ones [
74,
75]. Furthermore, hypoxia induces specific signaling pathways, bolstering glioma resistance to treatment through exosome absorption [
76]. According to Garnier et al. [
77], glioma stem cells (GSCs) release exosome-transcribed MGMT mRNA, reflecting TMZ resistance. Another study established that MGMT genomic rearrangements contribute to TMZ resistance by repairing the O6-Methylguanine lesion caused by TMZ [
78]. Moreover, research has highlighted the potential of the exosome-mediated circWDR62 and macrophage migration inhibitory factor (MIF) in increasing TMZ resistance in glioma, suggesting their value as prognostic biomarkers [
79,
80]. This also emphasizes the involvement of tumor macrophages in recurrent GBM [
81].
1.5. Exosomes in Mediating Immune Responses
Exosomes derived from tumors interact with various immune cells, including effector T cells, naturally occurring T
reg cells, and natural killer (NK) cells, contributing to immune suppression and tumor growth [
82]. Antigen-presenting molecules, TGF-b, tumor antigens, and immune intracellular adhesion molecules can all be identified in the serum exosomes separated from GBM patients [
83]. Brain tumor-initiating cells produce the extracellular matrix protein TNC associated with exosomes to limit T cell activity, as demonstrated in research by Reza and colleagues [
84]. TNC interacts with the α5β1 and αvβ6 integrins on T cells, leading to reduced mTOR signaling and halting T cell growth. Another study revealed that miR-1246 mediates H-GDE-induced M2 macrophage polarization by targeting TERF2IP, activating the STAT3 signaling pathway while hindering the NF-B signaling pathway, potentially creating an immunosuppressive microenvironment [
85]. A recent study found that circNEIL3, packaged into exosomes by hnRNPA2B1, is conveyed to infiltrated tumor-associated macrophages, allowing them to acquire immunosuppressive properties by stabilizing IGF2BP3, thereby promoting glioma progression [
86].
In summary, the bioactive molecules transported by exosomes play a significant role in glioma progression. It is important to note that the various stages of tumor development are interconnected and mutually influenced, rather than independent. Further exploration of the types and mechanisms of action of bioactive molecules influencing glioma progression is essential for their potential use as diagnostic and therapeutic tools.
2. Exosomes in Glioma Treatment
In recent years, research on exosomes for GBM treatment has yielded promising results as scientists gain a deeper understanding of their components’ roles. This research can be broadly categorized into two main directions:
2.1. Utilization of Inherent Characteristics of Exosomes
Exosomes exhibit characteristics influenced by the organ and tissue of their origin, granting them distinct properties, including tropism to specific organs and uptake by particular cell types [
24]. Notably, exosomes derived from human-umbilical-cord-derived mesenchymal stem cells (hUC-MSCs) have shown promise in partially inhibiting tumor growth by modulating the miR-10a-5p/PTEN pathway. Research conducted by Hao et al. suggests that hUC-MSCs-derived exosomes may offer alternative strategies for treating glioma [
126]. Furthermore, exosomes derived from rat bone marrow mesenchymal stem cells (rBMMSCs) have demonstrated their potential as a standalone treatment for glioblastoma (GBM), independent of additional therapies or as a drug delivery system, as revealed in recent investigations [
127]. Another study highlighted that exosomes derived from LPS/INFγ-triggered microglial cells have the ability to induce phenotypic shifts in tumor-associated myeloid cells. This shift is characterized by the upregulation of genes related to inflammation and leads to the inhibition of glioma growth [
128]. Additionally, the incorporation of focused ultrasound has been shown to enhance the efficacy of exosome accumulation in gliomas, thereby improving the effectiveness of the treatment course [
129].
Exosomes produced by cancer cells play a crucial role in driving overall tumor progression [
82]. Consequently, there is ongoing research into methods for eliminating or suppressing their production, often considered as supplementary therapies. One noteworthy target in this effort is Munc 13-4, a Ca
2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor and Rab-binding protein. Studies have demonstrated that Munc 13-4 serves as the key regulator for the Ca
2+ ion-mediated exosome release pathway in various cancer cell lines (although not specifically in glioma cells). Thus, targeted depletion of Munc 13-4 offers the potential to suppress oncogenic exosome secretion and hinder tumor progression [
130]. Furthermore, the secretion of exosome-related PTRF can be controlled through the ubiquitination of PTRF by UBE2O, leading to a downregulation of exosome release [
131]. Boosting UBE2O expression in cells holds promise as a novel approach for glioma treatment. In a similar vein, proton pump inhibitors (PPIs) and neutral sphingomyelinase 2 (nSMase2) are potential targets for reducing exosome secretion [
132,
133].
Exosome-mediated immunotherapy holds promise as an effective treatment approach, leveraging the unique properties of exosome donor cells [
134]. Dendritic cells (DCs) stand out as highly efficient antigen-presenting cells, capable of eliciting potent immune responses. In preclinical studies, DC-derived exosomes have demonstrated superior anticancer efficacy compared to traditional DC vaccinations. This superiority arises from their enhanced immunogenicity and increased resistance against immunosuppressive factors [
135,
136]. Ning et al. made a significant discovery by demonstrating that DC-derived exosomes, loaded with chaperone-rich cell lysates and modulating Cbl-b and c-Cbl signaling, can trigger more robust antitumor T-cell immune responses [
137]. Additionally, Liu et al. found that tumor-derived exosomes containing α-Galcer are effective for DC-based vaccinations. They employed α-Galcer-activated invariant NK-T cells as a cellular adjuvant, breaking immunological tolerance and generating antigen-specific cytotoxic T-cell (CTL) responses against GBM cells [
138]. Furthermore, a MUC1 glycopeptide antigen was recently linked to dendritic-cell-derived exosomes to create a promising anticancer vaccine candidate. This construct not only increased cytokine production in vivo but also induced high MUC1-specific IgG antibody titers with strong binding affinity for MUC1-positive tumor cells [
139]. Exosome-mediated immunotherapy has gained significant attention recently and exhibits remarkable potential as a treatment option for gliomas [
140].
2.2. Exosomes as Delivery Systems
Exosomes, owing to their biocompatibility, stability, and diminutive nano-scale dimensions, have been engineered to serve as effective carriers for therapeutic compounds, demonstrating promising initial success.
2.2.1. Chemotherapy Drugs as Exosome Payloads
Exosomes can serve as efficient carriers for delivering chemotherapy medications into the brain and glioma site, overcoming the restrictions of the blood–brain barrier. Studies have utilized brain-endothelial-cell-isolated exosomes loaded with paclitaxel and doxorubicin, demonstrating their potential to inhibit GBM growth [
141]. Similarly, coating doxorubicin-loaded nanoparticles with brain-endothelial-cell-derived exosomes allows for effective drug delivery to GBM cells, extending the survival of GBM-bearing animals [
142]. Neutrophil exosomes, due to their inflammation-driven nature and BBB-crossing ability, have also been employed for transporting doxorubicin, effectively targeting infiltrating GBM cells [
143].
Combining modified exosomes with other drugs, such as temozolomide, further enhances the efficacy of GBM therapy. Modified exosomes loaded with paclitaxel, created from embryonic stem cells and conjugated with a tumor-targeting peptide, have shown improved targeting and therapeutic effectiveness against GBM cells [
55]. In addition, exosomes were loaded with superparamagnetic iron oxide nanoparticles (SPIONs) and curcumin (Cur), and click chemistry was employed to attach a neuropilin-1-targeted peptide to the exosome membrane, thus creating glioma-targeting exosomes with combined imaging and therapeutic capabilities [
144]. Drawing inspiration from brain-targeting exosomes, Wang and colleagues reported the exploration of biomimetic nanovesicles [
145]. These were engineered through membrane fusion between blood exosomes and tLyp-1 peptide-modified liposomes, demonstrating potential for brain-targeted drug delivery. These examples underscore the potential of exosomes in drug delivery against brain tumors. More research and details are shown in
Table A2.
2.2.2. Nucleotide Drugs as Exosome Payloads
Nucleotide-based gene therapy holds great promise in clinical applications. Exosomes can potentially deliver mRNA, noncoding RNA, and antisense oligonucleotides (AON) to inhibit glioma. Studies have shown that exosomes loaded with miR-124a or miR29a-3p can significantly reduce the vitality and clonogenicity of GSCs [
146,
147]. Targeted modification of exosomes further enhances their delivery efficiency [
51]. These results demonstrate the potential of exosomes as delivery systems for anti-tumor nucleotide drugs, indicating their potential for therapeutic use. Before these treatment approaches can be implemented in clinical settings, further extensive research is needed.
This entry is adapted from the peer-reviewed paper 10.3390/cancers16040823