Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Today, recombinant adeno-associated virus (rAAV) vectors represent the vector systems which are mostly used for in vivo gene therapy for the treatment of rare and less-rare diseases. Although most of the past developments have been performed by using a transfection-based method and more than half of the authorized rAAV-based treatments are based on transfection process, the tendency is towards the use of stable inducible packaging and producer cell lines because their use is much more straightforward and leads in parallel to reduction in the overall manufacturing costs.
- stable inducible packaging and producer cell lines
- HeLa cells
- HEK293 cells
- CAP cells
- Sf9 cells
- rAAV
- adenovirus
- herpes simplex virus
- baculovirus
1. Stable Packaging and Producer Cell Lines
The starting point for the development of stable packaging and producer cell lines was the need to gain access to a scalable rAAV production, which is simpler and more straightforward than the transfection system based on the use of adherently grown HEK293 cells because large-scale production of rAAV vectors for clinical use was not feasible with the existing production protocol at the end of the 1990s.
In the late 1990s/early 2000s, the first scientific papers on the development of stable packaging and producer cell lines were published, which were in most cases based on the use of HeLa cells [1][2], although A549 cells have also been used [3].
Packaging and producer cell lines can be distinguished by the following characteristics. Whereas packaging cell lines contain only the rep/cap functions required for the production of AAV, producer cell lines contain the rep/cap functions as well as the rAAV vector construct (transgene expression cassette flanked by the two ITRs). In more recent developments, inducible producer cell lines have also contained the adenoviral helper functions. Figure 1 presents the development and the mode of AAV production using packaging and producer cell lines (HeLa or A549 cells), respectively.
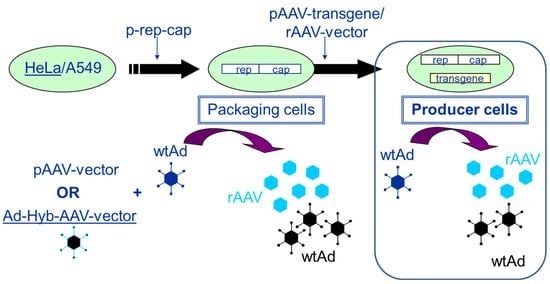
Figure 1. Establishment of packaging and producer cell lines for rAAV vector production. The transfection of HeLa or A549 cells with a plasmid harboring the rep2 (from AAV2)-capx (from any AAV serotype) sequences (p-rep-cap in the figure) leads to the establishment of a packaging cell line. When these cells are either transfected with a pAAV-vector (plasmid) followed by infection with an helper virus (wtAd) or infected with adenovirus (wtAd) and 24 h later with an E1-deleted adenovirus—AAV-Hybrid virus (providing the rAAV transgene sequence flanked by the two ITRs) (Ad-Hyb-AAV-vector)—rAAV as well as adenovirus are produced. If the rAAV transgene sequence is stably integrated via plasmid transfection (pAAV-transgene) or vector transduction (rAAV-vector), the packaging cells become stable producer cells. Upon infection with adenovirus (wtAd), these cells start to produce rAAV vector and adenovirus (wtAd).
Earlier in the 2000s, the Sf9 baculovirus system was developed for the production of AAV [4], which was initially based on the infection of Sf9 cells with recombinant baculoviruses. However, in order to also improve this production system and reduce the manufacturing costs, packaging cells have been developed which are going to be used for routine manufacturing of rAAV vectors (see below).
2. HeLa- and A549-Based Packaging and Producer Cell Lines
2.1. HeLa Cell-Based Packaging Cell Lines
In order to induce vector production with packaging cell lines containing the rep/cap functions, the rAAV vector construct can be produced either via transfection or via infection by an adenovirus–AAV hybrid virus (preferable) and infection by the adenoviral helper virus. In general, authors have used a sequential infection scheme of the packaging cell lines, initiating this infection with the adenoviral helper virus with functional genes of the E1 region and subsequently (e.g., 24 h later) with an adenovirus-AAV hybrid virus containing the rAAV transgene sequence in the E1 region of the adenovirus [5]. This sequential infection is required because it allows for the production of sufficient rep proteins due to the activation of the AAV p5, p19, and p40 transcription units of the rep-cap genes by the adenoviral helper virus before replication of the E1-deficient rAd genome is initiated.
All studies have established a strong correlation between rep/cap gene amplification and a high yield of rAAV production [6][7][8].
Although a wt adenovirus can be used for the initial infection, it is preferable that this adenovirus is replication deficient in order to avoid its co-production in parallel to AAV and contamination of the final AAV vector product. In this context, adenoviral mutants characterized by a temperature-sensitive E2b gene (sub100r [9] or ts149 [7]) can be used. Furthermore, the adenovirus–AAV hybrid virus is preferentially E1 negative in order to preclude its production during the AAV production and, thus, contamination of the final AAV vector preparation.
The advantages of the packaging cell line-based production systems are the fact that these systems are rather versatile and the production of new vector/new transgene constructs can be easily performed via the modification of the adenovirus–AAV hybrid-virus. Further advantages are the 5–10-fold higher vector productivity in comparison to the transfection based production method [10], as well as the fact that no rcAAV (replication competent AAV)/wtAAV is generated in parallel to rAAV production (<1 rcAAV particle/109 AAV vector particles) [9][10]. The generation of rcAAV is a general problem observed in the transient transfection-based production system because of potential non-homologous recombination events during transfection [11][12].
However, some drawbacks should also be mentioned here: in order to avoid the production of adenovirus helper virus-free AAV batches, temperature-sensitive strains have to be used, which unfortunately are prone to reversions and are thus difficult to produce and characterize. Furthermore, in order to produce AAV with this production method, the packaging cells have to be infected with two different viruses, which signifies that, for a GMP process, two different adenoviruses have to be produced under GMP conditions and undergo a QC release testing. For this reason, large-scale productions are preferably performed using producer cell lines because only one adenovirus is required for inducing the production of AAV vectors (mono-infection in contrast to a staggered infection with two adenoviruses).
In the context of the use of packaging cell lines, the recently published TESSA (Tetracycline-Enabled Self-Silencing Adenovirus) should be mentioned. This system that was initially developed for an infection-based rAAV production system is based on the following modifications of the adenoviral helper viruses [13]: the helper adenovirus self-inhibits its major late promoter (MLP) to truncate its own replication. The insertion of a tetracycline repressor (TetR) binding site into the MLP and encoding of the TetR under its transcriptional control allows for normal virus replication in the presence of doxycycline but only for genome amplification and the expression of early genes (the helper functions) in its absence. By co-infecting HEK293 cells with two TESSAs, one coding for AAV rep and cap genes and the second one for the rAAV genome, the two TESSAs deliver adenoviral helper functions in addition to the AAV rep and cap genes and the rAAV genome. Up to 30-fold-more rAAV vectors were obtained compared to the helper virus-free plasmid approach. Cell-specific production levels of >105 gc/cell have been reported. This production system has significantly improved particle infectivity (5–60 fold) as shown for rAAV2, 6, 8, and 9. Moreover, the 99.99992% reduction of adenovirus generation compared to normal adenovirus production is a further advantage because of the very low contamination of the rAAV preparation by the helper virus.
In 2023, the same authors [66] published an adaptation of the TESSA system to use in the context of packaging cell lines containing the rep and cap genes (HeLa RC32). The original TESSA system enables rAAV production only when the rep and cap genes are provided in trans, but not from stable packaging cells. Using HeLa RC32, the authors have shown that expression of the adenovirus L4 22/33K unit is essential for rep/cap amplification but that the proteins are titrated away by binding to replicating adenovirus genomes. siRNA-knockdown of the adenovirus DNA polymerase or the use of a thermosensitive TESSA mutant (TESSA-E1-tsDNA) decreased adenovirus genome replication whilst maintaining MLP repression. In this study, the HeLa RC32 cells were either transfected with the pAAV transgene plasmid or infected with rAAV transgene in parallel to infection with modified TESSA. Thus, this paper presented the development of the TESSA system towards the use for rAAV production using packaging cells; however, further modifications are still required to render this system of interest for the industrial production of rAAV. Amongst other issues, it will be necessary to deliver the rAAV genome directly via TESSA and not separately via transfection or rAAV infection. However, in fine, this system is of high interest because it is an infection-based system which is always more efficient than the transfection-based production system [59].
The development of a packaging cell line was in many cases also the intermediate step towards the establishment of a producer cell line.
2.2. HeLa Cells versus A549 Cells
In 1998, Gao et al. [9] published the development of HeLa-based packaging cells. Clones were generated by transfecting HeLa with plasmids containing the rep/cap helper functions under the control of AAV promoters. A number of 708 clones were selected, of which 515 survived expansion. However, only eight of these clones were able to trans-complement rep/cap. The best clone, called B50, was further evaluated. In order to induce the production of AAV vectors, the cells were infected with an adenovirus defective in E2B to induce the expression of the rep and cap proteins, which was followed by infection (24 h later) with a replication-defective hybrid adenovirus in which the AAV vector construct was cloned into the E1 region. This infection led to a 100-fold amplification and rescue of the AAV genome, leading to a high yield of recombinant AAV, free of replication-competent AAV; 3.3 × 108 transducing units and 6.4 × 1012 genome copies were obtained per 109 cells.
Since HeLa cells contain sequences of the human papilloma virus, the same group evaluated the A549 cells using the same approach [14]. A549 cells were derived initially from a human alveolar cell carcinoma without any viral-mediated transformation [15]. A549 cells were transfected with the P5 rep/cap and clones were selected. One clone (K209) led to a 1000-fold amplification of the rep/cap genes and a high-level expression of AAV vectors upon infection with adenovirus. The required adenoviral MOI was 5–10-times lower than that required for the induction of AAV production by the HeLa-based packaging clone [9][14]. The vector yield per cell was comparable to that obtained with B50 cells, meaning that both cell lines can be used for the production of AAV; however, there is an advantage for the A549 cells because a lower MOI of adenovirus is necessary for infection. Furthermore, these results also indicate that the presence of papilloma viral factors present in HeLa cells are not required for AAV production.
It should also be mentioned here that only those clones that reached a level of >3000 copies of rep genes per cell produced high levels of AAV vectors [14].
2.3. Producer Cell Lines
AAV producer cell lines contain the rep/cap helper functions as well as the rAAV vector construct. Their development can be sequential, starting from a packaging cell line into which the rAAV vector construct is either introduced via stable transfection [2][6][16][17] or via transduction with the rAAV vector [3][18]. In the case of plasmid transfection, the development of such a cell line can also be performed in the form of a ‘one shot’, meaning that all required functions are inserted into the producer cell line at the same time [2][19]. The advantage of plasmid transfection relates to the possibility to select transfected cells via a resistance marker cloned into the plasmid. In the case of transduction using an rAAV vector (up to three rounds of transduction at 105 vp/c were used), the selection process is based on PCR screening and rAAV vector-production capacity induced by adenovirus infection can be rather cumbersome [3]. Martin et al. [19] reported that less than 6% of all cells analyzed are high-rAAV producer cells, meaning that a majority of the cell clones are non-producers. This also signifies that a large number of clones have to be analyzed.
For inducing AAV production in producer cell lines, the producer cells are infected at optimal cell density with the adenoviral helper virus, which is either replication competent or replication incompetent (see also packaging cells). The use of a replication-competent adenovirus for inducing rAAV production is straightforward and easier to produce than a replication-incompetent adenovirus; however, it has to be kept in mind that adenovirus is co-produced with the rAAV vector and has to be removed during DSP. Furthermore, for clinical vector productions, the removal and inactivation of the helper adenovirus has to be validated via a clearance study [1][20] (see below). In order to alleviate this drawback, Farson et al. [3] have used a replication-incompetent adenovirus; however, these viruses are sometimes characterized by instabilities [16] and, often, the obtained rAAV titers are lower than when replication-competent adenoviruses are used [21].
The used adenoviral MOI ranges from 10 to 100 and leads to considerable amplification of the rep and cap genes (by a factor of 100) [6]. Higher MOIs (optimum: 100) are required in order to ensure a sufficient rep-cap amplification, which directly impacts AAV vector yields [6].
3.2. HEK293- and CAP-Based Packaging and Producer Cell Lines
The use of HEK293 cells as base for the development of rAAV packaging and producer cell lines is less straightforward because the constitutive expression of the E1A gene, a REP transcription activator, leads to the expression of the cytostatic rep proteins (via activation of the p5 promoter). This signifies that HEK293 cells can only be used in conjunction with an induction system for the development of stable packaging/producer cell lines (as already indicated).
The first HEK293-based rAAV producer cell line was developed by Qiao et al. [22]. The authors developed a helper virus-free inducible producer cell line containing the sequences of the rAAV vector construct, the cap genes, as well as the four rep gene sequences, which were conditionally disrupted by insertion of an intron that harbors transcription-termination sequences flanked by the LoxP sites into their shared coding region. The promoters of the rep gene proteins are not affected; however, the presence of this intron leads to a premature transcription stop of rep transcription (Figure 2A). Upon infection by an adenovirus (MOI = 5) deleted for E1A, E1B, and E3 and carrying the cre gene, these producer cells started to generate high titers of AAV vectors (in this specific case with GFP as transgene) due to the reactivation of the transcription of all four rep proteins. This switch system was initially tested with the lacZ gene, and a 600-fold induction of β-galactosidase activity was observed. Finally, the authors evaluated this induction system for rAAV coding for other transgenes. The generated HEK293-based producer cell lines showed normal growth characteristics, high stability, and high yields of rAAV vectors. These producer cell lines did not produce rcAAV—no rcAAV particle could be detected in up to 109 viral genome particles. The cells produced about 50% of full rAAV particles with a significantly higher infectivity when compared to the triple-transfection method and a HeLa cell-based packaging cell line.
The main drawback of this producer cell line was the fact that the development of these cells required multiple cloning steps for the vector and packaging plasmids, and a two-step transfection and selection for stable cell lines. Thus, based on this approach, the same group [23] simplified the development of such producer cells by a one-step cloning of AAV vector cassette into the serotype-specific packaging plasmid and a single-plasmid transfection and selection for stable AAV vector producer cell lines (the cloning procedure was streamlined by using the Gateway technology). Upon infection with an E1A/E1B-deleted helper adenovirus, these producer cells produced high yields of different AAV serotypes (2, 8, 9): 5–8 × 1013 vg per Nunc Cell factory (0.9–1.3 × 105 vg/cell). The different clones contained 10–50 rep/cap gene copies/cell, and upon infection the copy number was amplified about 10–20-fold. Although rAAV production was performed with adherently grown HEK293 cells, the switch to serum-free suspension culture should not be too difficult because HEK293 cells can easily be adapted to suspension growth.
Very recently, Shape Therapeutics developed a stable rAAV producer cell line using a tet-on based Cre-Lox approach presented at the ECI Meeting on Advancing manufacture of cell and gene therapies VIII, Coronado/CA, Feb. 4-8, 2024): 'Genetic components required for AAV production are expressed in an inducible manner from three plasmids that are that are stably integrated into the genome of a human cell line. The addition of doxycycline drived the expression of a self-excising hormone-inducible cre recombinase (ER2 Cre). The floxed ER2 Cre and two floxed stuffer fragments block the expression of adenoviral E2A, E4orf6, and transcriptionally inactive VA RNA and AAV rep-cap genes, such that in the uninduced state, the resistance cassettes and the transgene are the only expressed genetic elements. The addition of doxycycline and tamoxifen causes activation and subsequent nuclear translocation of ER2 Cre which excises the floxed fragments, triggering the expression of adenoviral helper genes and the AAV rep-cap genes. This causes amplification of ITR flanked GOI (gene of interest) and its subsequent packaging into preformed capsids, thus making rAAV particles' (Pande S, Johnson C, Villela C, Bi T, Engelbert B, Saleem R, Prentice K; poster #61 (TruStable™: a fully integrated inducible stable producer cell line for AAV production). The real advantage of this system is that no helper virus is necessary any more. A further advantage is that a two step manufacturing process can be established with the first step destined to the generation of an elevated highly viable cell concentration which in the second step is induced to produce rAAV vectors. An intensified production process (10L) is able to produce a vector titer of 8E+11 vg/ml at a cell density of 10E+6 c/ml.
In 2023, another inducible HEK293-based rAAV producer cell line was published [24]. These cells contain the inducible rep gene constructs, the sequences for the expression of the cap proteins, as well as adenoviral helper genes (E2A, E4, VA-RNA). The expression of rep40 and rep68, inserted into the HEK293SF cells using lentiviral vectors, was under the control of two inducible promoters with different expression levels (higher expression levels for rep40 and lower expression level for rep68). The expression was induced by the addition of cumate/coumermycin.
Three of the established clones produced vector levels comparable to the triple-transfection process; the vector-production levels were very similar for rAAV2 and rAAV DJ. Furthermore, the clones were stable in continuous cultivation for up to 7 weeks. However, it should be mentioned here that during later passages, a reduction in the rep68 levels was observed, leading to a reduction in vector titers. This reduction in the expression was probably caused by an epigenetic silencing mechanism. Thus, this rAAV production system is not yet ready for large-scale use.
Based on previous studies [25], Lu et al. [26] have developed another inducible HEK293-based rAAV producer cell line by optimizing helper constructs and reducing the expression of the transgene during vector production. The different functions were placed on three separate plasmids which were transfected into HEK293 cells to generate stable rAAV producer clones. These plasmids contain the following functions (Figure 2B): the rAAV vector plasmid contains the rAAV vector construct flanked by the two ITRs with the expression of the transgene driven by the RSV-LacO promoter. The vector construct was cloned into a transposon backbone with a lacI repressor gene linked to a puromycin-resistance gene driven by a phosphoglycerate kinase promoter, thus leading to an inducible rAAV genome construct. The second transposon construct (the so-called replication module) provides the large rep protein (rep68) tagged with a mutant destabilization domain (FKBP12) as well as the adenoviral (Ad2) helper proteins (E4orf6, DBP), whose expressions are controlled by the Tet-on-based induction system. Furthermore, this replication module contains a hygromycin-resistance gene linked via T2A to the reverse Tet transactivator. The third transposon construct (the so-called packaging module) provides the sequences for expressing the capsid proteins as well as the small rep protein (rep52), whose expressions are controlled by a cumate switch. Although these cells already produce better titers than the initially developed cells [25], only the addition of the proteasome inhibitor MG132 for reducing the host cell-initiated degradation of rAAV allowed for the production of rAAV levels comparable to the triple-transfection system. Furthermore, 105 capsids and >104 vg are produced per cell. With respect to the level of full particles, they ranged between 25% and >50%.
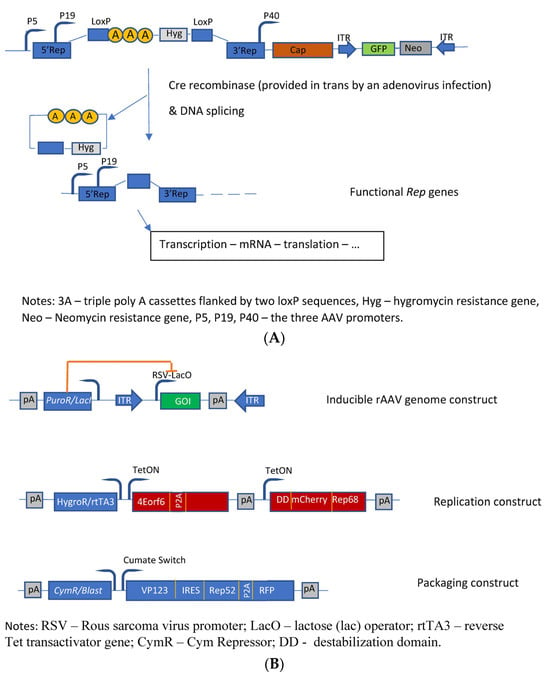
Figure 2. Molecular constructs used for the development of the different HEK293 cell based packaging/producer cell lines. (A) Developments performed by Qiao et al. [22]: Construction of dual-splicing switch AAV packaging plasmid; the switch system is based on the Cre-Lox system; (B) Constructs used for the development of an AAV producer cell line (synthetic cell line approach) [26].
In view of improved vector production, the authors proposed a further reduction in the expression of the transgene during vector production (e.g., use of tissue specific promoters whose choice depends on the target tissue to be transduced) as well as an increase in the expression of capsid proteins combined with an improved efficiency of viral genome packaging into capsids. A further improvement in production might be achieved via an exploration of other adenoviral helper components or their combination.
Finally, CEVEC (part of Cytiva) has developed inducible rAAV producer cell lines based on the use of HEK293 and CAP [79,80] cells. The so-called Alpha cell line contains all the required (helper) functions (adenoviral helper functions: E2A, E4orf6, VA RNA; the AAV rep functions), which are inducible upon the addition of doxycycline (Tet-on induction system). In the second step, the desired serotype-specific cap sequence as well as rAAV vector sequence are stably integrated in order to obtain to the specific rAAV producer cell line. Genome integration of the rep gene was possible because of the inactivation of the p19 promoter while the functionality of Rep78/68 was maintained [81]. A clonal stability of over 75 passages was communicated. This production system was developed in view of large-scale industrial production of rAAV for clinical and commercial purposes (→ELEVECTA AAV production platform). RAAV production processes have been scaled to 200 L. When inducing rAAV production at a cell density of 5–6 × 106 c/mL, the cell culture produces vector titers of up to 5.7 × 1013 vg/L (6 days after induction) [82]. Further optimization of the cell culture process towards a high cell density perfusion system allowed for obtention of 2 × 107 c/mL and an rAAV production level of >1015 vg/L with about 30% of full particles [83].
3. Insect Cell-Based Packaging Cell Lines
As mentioned above, another approach for producing large amounts of rAAV vectors is the Sf9/baculovirus system. The original baculovirus system was based on the infection of Sf9 cells with three baculoviruses [27], followed by optimization in order to improve the expression system but also for reducing the number of baculoviruses via the development of the dual baculovirus system [28] and the Monobac system [29].
As mentioned, the reduction in the number of baculoviruses was necessary, firstly for reducing the number of different viruses to generate for production purposes, but also for ensuring that all Sf9 cells received the required functions for producing rAAV.
In addition to the Monobac system [29], another approach is the development and optimization of stable producer cell lines, which is presented in the following.
The initial development was published by Aslanidi et al. [30]. The authors developed a two-component system consisting of one recombinant Sf9 cell line and a recombinant baculovirus. The recombinant Sf9 cell line contained silent copies of the rAAV rep and cap genes (Figure 3A). Both genes were controlled by the very late baculoviral polyhedrin promoter and the cis-acting enhancer element hr2-0.9. Upon infection with baculovirus harboring the rAAV genome, the hr2.09 enhancer was transactivated by the baculoviral IE-1 gene, leading to the expression of the rep and cap genes of AAV. A feed-forward loop was initiated by the induced Rep78 protein, boosting the amplification of the integrated genes by interaction with the incorporated cognate AAV rep-binding site (RBE) because the P5 RBE mediated the amplification of integrated AAV sequences in mammalian cells [31], thus dramatically improving yields of rAAV production, as shown for HeLa-based packaging cell lines [31]. Furthermore, it was also shown previously that either AAV ITR or P5 RBE modulates the expression from the P19 promoter [32]. In the system developed by Aslanidi et al. [30], the result was an elevated production of rAAV which could exceed the production levels obtained by the triple-transfection method by 10 fold. These packaging cell lines have been evaluated for the production of AAV serotypes 1 and 2. A further advantage of this system is the genetic stability of these cells.
Mietzsch et al. [33] further developed this system to the OneBac 1.0 platform for the generation of all AAV serotypes ranging from 1 to 12 (Figure 3B). Specific production rates of up to 5 × 105 infectious particles per cell (AAV3) were obtained.
Although these packaging cells are of high interest because of the simplification of the insect cell/baculovirus-based production system, unfortunately they are affected by an important disadvantage. The collateral packaging of helper DNA into AAV capsids was observed. This disadvantage was tackled by further developments performed by Mietzsch et al. [34].
Mietzsch et al. [34] reported considerable co-packaging of rep, cap, bsd, and hr2 sequences into rAAV5 capsids, e.g., reaching up to 35.6–55.3% of particles positive for the cap sequence. They have shown that the removal of the RBE sequence from cap and rep constructs in the recombinant Sf9 cells (Figure 3C) led to a considerable reduction in the contamination of rAAV capsids with cap-positive AAV capsids at a level of 0.02–0.03%, but this removal also proved that baculovirus-induced AAV rep/cap template amplification is partially independent of the rep–RBE interaction and, thus, not necessary for rAAV production for this production system. Further optimization of the cap sequence construct (splicing-based strategy to raise the relative amounts of VP1 in AAV5 capsids) led to AAV5 infectivity exceeding that of Sf9 or HEK293 cell-based AAV5 production (=development of the OneBac 2.0 system).
The general drawback of the OneBac systems is the fact that, for each new serotype, a new producer cell line has to be generated. A solution was published by Wu et al. [35], who established a packaging cell line which provides only the rep functions, whereas the baculovirus required for the induction of the production of the rAAV vector brings in the cap gene of the desired serotype as well as the rAAV genome (ITR-GOI) sequence (=dual functional BEV-CAP-(ITR-GOI)) (Figure 3D). Furthermore, they used a p10 promoter to regulate cap gene expression in the baculovirus transactivator, which resulted in lower promoter–promoter interaction (the expression of the rep proteins was under control of the polyhedrin promoter). Although the novel Sf9-GFP/rep packaging cell line-dependent OneBac system is versatile, flexible, and maintains high virus yields, the main drawback is the fact that the rep gene construct is inserted into the packaging cell line as performed by Mietzsch et al. [34] for the OneBac 1.0 system. As already mentioned above, the presence of the RBE sequence in the construct will still lead to the encapsidation of rep sequences into a certain percentage of rAAV particles. With respect to rAAV vector production, this modified OneBac system had the following features: the cells were stable for up to 10 passages, and after infection with rec. baculovirus they showed a specific vector productivity of >105 vg/cell. The serotypes AAV2, AAV8, and AAV9 were produced with this system and the ratio of the capsid proteins had the expected ratio of ~1:1:10 (vp1:vp2:vp3).
Finally, the OneBac system was further optimized by Moreno et al. [36]. The authors kept the separation of the rep gene from the cap gene by providing the latter gene combined with the rAAV vector construct on one rec. baculovirus (e.g., polH-Cap-ITR-transgene-ITR), more or less similar to that developed by Wu et al. [35]. Concerning the rep gene, a stable Sf9 cell line was constructed containing the inducible rep gene (Figure 3E). This article was mainly focused on the development of this rep expression cassette. The optimization concerned the choice of baculovirus hr sequence, the separation of the large and small rep sequences (comparable to Urabe’s approach [27]), and the use of different promoters for driving the expression of the rep proteins. The use of different promoters was chosen in order to avoid promoter interaction related to the expression of the rep and cap genes and, on the other hand, to obtain a more sequential expression of the rep proteins. The choice of a double-rep cassette design allowed for an efficient and timely control of rep expression following the expression dynamics of wild-type AAV rep expression. In mammalian cells, the large rep proteins have to be expressed earlier than the small rep proteins because at first rAAV vector DNA has to be replicated (which requires the presence/activity of the large rep proteins). Later on, the small rep proteins are required because they are mainly responsible for the accumulation and packaging of the single-strand vector DNA. Thus, the authors have chosen the 39k promoter, an immediate early promoter, which is transactivated 3–6 h post-baculovirus infection, for driving the expression of the large rep proteins and the polH promoter, a late baculovirus promoter (or p10 or p6.9), to regulate the majority of expression of the small rep proteins in the double-rep cassette BEV system. This approach led to an increased full-to-empty AAV particle ratio. As mentioned, the authors have also evaluated the use of other baculovirus hr enhancer sequences. The original OneBac system used the hr2.09 enhancer, which shows a relatively high basal expression, whereas some alternative enhancer sequences (hr4b, hr5) show reduced basal expression. The authors have shown that all the tested hr sequences enhanced baculovirus promoter activity upon transactivation with baculoviruses, and both enhancers (hr4b and hr5) were used in the double-rep cassette in conjunction with the cassette encoding for the large rep proteins and the small rep proteins (in the inverse direction), respectively.
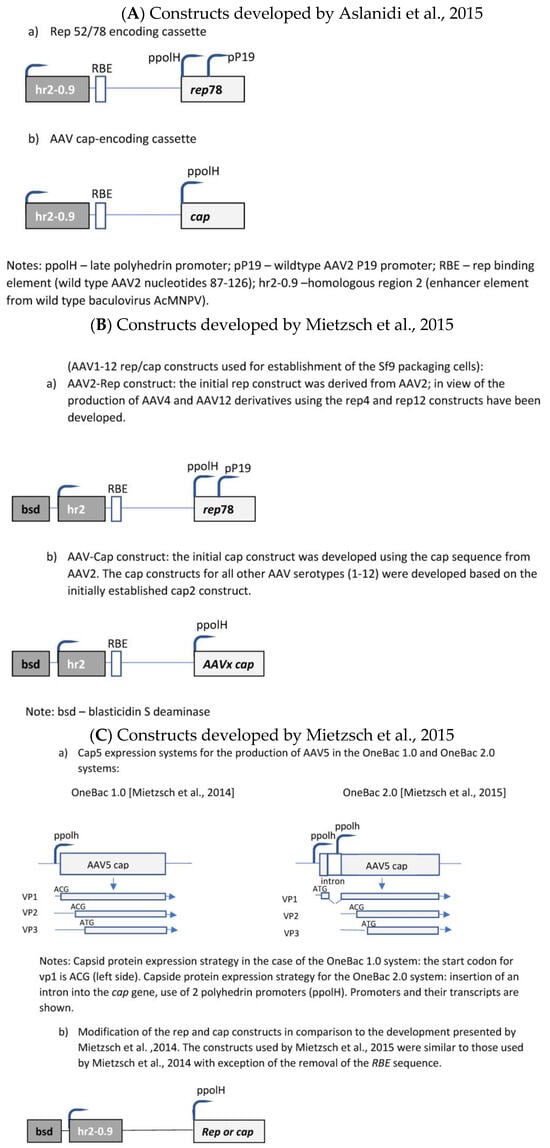
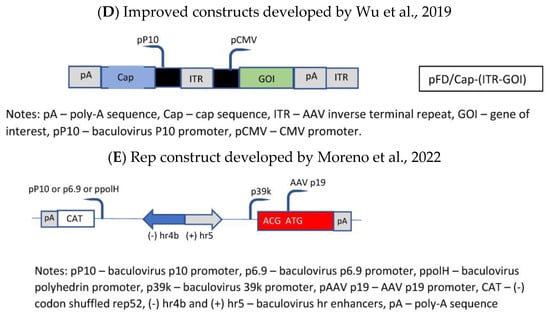
Figure 3. Molecular constructs used for the development of the different insect cell based packaging cell lines. (A) Constructs developed by Aslanidi et al. [30]; (B) Constructs developed by Mietzsch et al. [33] in the frame of the OneBac development (AAV1-12 rep/cap constructs used for establishment of the Sf9 packaging cells); (C) Constructs developed by Mietzsch, et al. [34] in the frame of the OneBac 2.0 development; (D) Wu, et al. [35] did not modify the rep construct but developed a combined cap—rAAV construct in order to dispose of a flexible system for easily switching from one AAV serotype to another one and from one transgene construct to another one. Thus, the rep construct was similar to that used by Mietzsch et al. [33]; (E) Rep construct developed by Moreno et al. [36].
This system allows for the production of at least 1011 gc/mL, with a specific productivity of 105 gc/cell. The final evaluations were performed in 2 L reactors. Furthermore, the authors have shown that the cells were stable for nine passages in the absence of antibiotic selection.
Finally, it should also be mentioned here that because of the way the rep and cap constructs have been developed in frame of the Sf9/baculovirus system, no rcAAV generation is to be expected.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms12020384
References
- Thorne, B.A.; Takeya, R.K.; Peluso, R.W. Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Hum. Gene Ther. 2009, 20, 707–714.
- Clark, K.R.; Voulgaropoulou, F.; Fraley, D.M.; Johnson, J.P. Cell lines for the production of recombinant adeno–associated virus. Hum. Gene Ther. 1995, 6, 1329–1341.
- Farson, D.; Harding, T.C.; Tao, L.; Liu, J.; Powell, S.; Vimal, V.; Yendluri, S.; Koprivnikar, K.; Ho, K.; Twitty, C.; et al. Development and characterization of a cell line for large-scale, serum-free production of recombinant adeno-associated viral vectors. J. Gene Med. 2004, 6, 1369–1381.
- Kotulska, K.; Fattal-Valevski, A.; Haberlova, J. Recombinant adeno-associated virus serotype 9 gene therapy in spinal muscular atrophy. Front. Neurol. 2021, 12, 726468.
- Liu, X.L.; Clark, K.R.; Johnson, P.R. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 1999, 6, 293–299.
- Liu, X.; Voulgaropoulou, F.; Chen, R.; Johnson, P.R.; Clark, K.R. Selective Rep-Cap gene amplification as a mechanism for high-titer recombinant AAV production from stable cell lines. Mol. Ther. 2000, 2, 394–403.
- Tessier, J.; Chadeuf, G.; Nony, P.; Avet-Loiseau, H.; Moullier, P.; Salvetti, A. Characterization of adenovirus-induced inverted terminal repeat-independent amplification of integrated adeno-associated virus rep-cap sequences. J. Virol. 2001, 75, 375–383.
- Gao, G.; Wilson, J.M.; Wivel, N.A. Production of recombinant adeno-associated virus. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Shatkin, A.J., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 529–544.
- Gao, G.P.; Qu, G.; Faust, L.Z.; Engdahl, R.K.; Xiao, W.; Hughes, J.V.; Zoltick, P.W.; Wilson, J.M. High-titer adeno–associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 1998, 9, 2353–2362.
- Zhang, H.; Xie, J.; Xie, Q.; Wilson, J.M.; Gao, G. Adenovirus—Adeno-Associated virus hybrid for large-scale recombinant Adeno-Associated Virus production. Hum. Gene Ther. 2009, 20, 922–929.
- Salvetti, A.; Orève, S.; Chadeuf, G.; Favre, D.; Cherel, Y.; Champion-Arnaud, P.; David-Ameline, J.; Moullier, P. Factors influencing recombinant adeno-associated virus production. Hum. Gene Ther. 1998, 9, 695–706.
- Allen, J.M.; Debelak, D.J.; Reynolds, T.C.; Miller, A.D. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J. Virol. 1997, 71, 6816–6822.
- Su, W.; Patrício, M.I.; Duffy, M.R.; Krakowiak, J.M.; Seymour, L.W.; Cawood, R. Self-attenuating adenovirus enables production of recombinant adeno-associated virus for high manufacturing yield without contamination. Nat. Commun. 2022, 13, 1182.
- Gao, G.P.; Lu, F.; Sanmiguel, J.C.; Tran, P.T.; Abbas, Z.; Lynd, K.S.; Marsh, J.; Spinner, N.B.; Wilson, J.M. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol. Ther. 2002, 5, 644–649.
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423.
- Blouin, V.; Brument, N.; Toublanc, E.; Raimbaud, I.; Moullier, P.; Salvetti, A. Improving rAAV production and purification: Towards the definition of a scaleable process. J. Gene Med. 2004, 6 (Suppl. S1), S223–S228.
- Toublanc, E.; Benraiss, A.; Bonnin, D.; Blouin, V.; Brument, N.; Cartier, N.; Epstein, A.L.; Moullier, P.; Salvetti, A. Identification of a replication-defective herpes simplex virus for recombinant adeno-associated virus type 2 (rAAV2) particle assembly using stable producer cell lines. J. Gene Med. 2004, 6, 555–564.
- Matthews, L.C.; Gray, J.T.; Gallagher, M.R.; Snyder, R.O. Recombinant adeno-associated viral vector production using stable packaging and producer cell lines. Methods Enzymol. 2002, 346, 393–413.
- Martin, J.; Frederick, A.; Luo, Y.; Jackson, R.; Joubert, M.; Sol, B.; Poulin, F.; Pastor, E.; Armentano, D.; Wadsworth, S.; et al. Generation and characterization of Adeno-Associated Virus producer cell lines for research and preclinical vector production. Hum. Gene Ther. 2013, 24, 253–269.
- Thorne, B.A.; Quigley, P.; Nichols, G.; Moore, C.; Pastor, E.; Price, D.; Ament, J.W.; Takeya, R.K.; Peluso, R.W. Characterizing clearance of helper adenovirus by a clinical rAAV1 manufacturing process. Biologicals 2008, 36, 7–18.
- Jenny, C.; Toublanc, E.; Danos, O.; Merten, O.-W. Evaluation of a serum-free medium for the production of rAAV-2 using HeLa derived producer cells. Cytotechnology 2005, 49, 11–23.
- Qiao, C.; Wang, B.; Zhu, X.; Li, J.; Xiao, X. A novel gene expression control system and its use in stable, high-titer 293 cell-based adeno-associated virus packaging cell lines. J. Virol. 2002, 76, 13015–13027.
- Yuan, Z.; Qiao, C.; Hu, P.; Li, J.; Xiao, X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011, 22, 613–624.
- Jalšić, L.; Lytvyn, V.; Elahi, C.M.; Hrapovic, S.; Nassoury, N.; Chahal, P.S.; Gaillet, B.; Gilbert, R. Inducible HEK293 AAV packaging cell lines expressing Rep proteins. Mol. Ther. Methods Clin. Dev. 2023, 30, 259–275.
- Lee, Z.; Lu, M.; Irfanullah, I.; Soukup, M.; Hu, W.-S. Construction of an rAAV producer cell line through synthetic biology. ACS Synth. Biol. 2022, 11, 3285–3295.
- Lu, M.; Lee, Z.; Lin, Y.-C.; Irfanullah, I.; Cai, W.; Hu, W.-S. Enhancing the production of recombinant adeno-associated virus in synthetic cell lines through systematic characterization. Biotechnol. Bioeng. 2024, 121, 341–354.
- Urabe, M.; Ding, C.; Kotin, R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002, 13, 1935–1943.
- Smith, R.H.; Levy, J.R.; Kotin, R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009, 17, 1888–1896.
- Galibert, L.; Jacob, A.; Savy, A.; Dickx, Y.; Bonnin, D.; Lecomte, C.; Rivollet, L.; Sanatine, P.; Boutin Fontaine, M.; Le Bec, C.; et al. Monobac system—A single baculovirus for the production of rAAV. Microorganisms 2021, 9, 1799.
- Aslanidi, G.; Lamb, K.; Zolotukhin, S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl. Acad. Sci. USA 2009, 106, 5059–5064.
- Nony, P.; Tessier, J.; Chadeuf, G.; Ward, P.; Giraud, A.; Dugast, M.; Linden, M.; Moullier, P.; Salvetti, A. Novel cis-acting replication element in the Adeno-Associated Virus type 2 genome is involved in amplification of integrated rep-cap sequences. J. Virol. 2001, 75, 9991–9994.
- Lackner, D.F.; Muzyczka, N. Studies of the mechanism of transactivation of the adeno-associated virus p19 promoter by Rep protein. J. Virol. 2002, 76, 8225–8235.
- Mietzsch, M.; Grasse, S.; Zurawski, C.; Weger, S.; Bennett, A.; Agbandje-McKenna, M.; Muzyczka, N.; Zolotukhin, S.; Heilbronn, R. OneBac: Platform for scalable and high-titer production of Adeno-Associated Virus Serotype 1–12 vectors for gene therapy. Hum. Gene Ther. 2014, 25, 212–222.
- Mietzsch, M.; Casteleyn, V.; Weger, S.; Zolotukhin, S.; Heilbronn, R. OneBac 2.0: Sf9 cell lines for production of AAV5 vectors with enhanced infectivity and minimal encapsidation of foreign DNA. Hum. Gene Ther. 2015, 26, 688–697.
- Wu, Y.; Mei, T.; Jiang, L.; Han, Z.; Dong, R.; Yang, T.; Xu, F. Development of versatile and flexible Sf9 packaging cell line-dependent OneBac system for large-scale recombinant adeno-associated virus production. Hum. Gene Ther. 2019, 30, 172–183.
- Moreno, F.; Lip, F.; Rojas, H.; Anggakusuma. Development of an insect cell-based adeno-associated virus packaging cell line employing advanced Rep gene expression control system. Mol. Ther. Methods Clin. Dev. 2022, 27, 391–403.
This entry is offline, you can click here to edit this entry!
