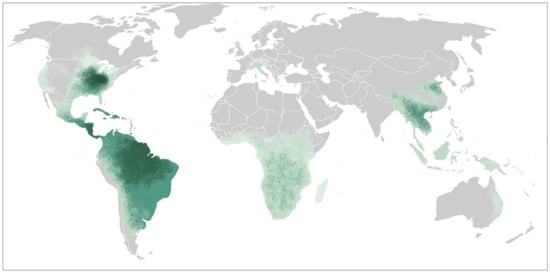
Histoplasma capsulatum, the etiological agent for histoplasmosis, is a dimorphic fungus that grows as a mold in the environment and as a yeast in human tissues. It has a broad global distribution with shifting epidemiology. While in immunocompetent individuals infection is usually self-resolving, solid organ transplant recipients are at increased risk of symptomatic disease with dissemination to extrapulmonary tissue. Diagnosis of histoplasmosis relies on direct observation of the pathogen (histopathology, cytopathology, and culture) or detection of antigens, antibodies, or nucleic acids. All transplant recipients with histoplasmosis warrant therapy, though the agent of choice and duration of therapy depends on the severity of disease.
- histoplasmosis
- solid organ transplant
- transplant
- Histoplasma
1. Introduction
2. Pathogenesis
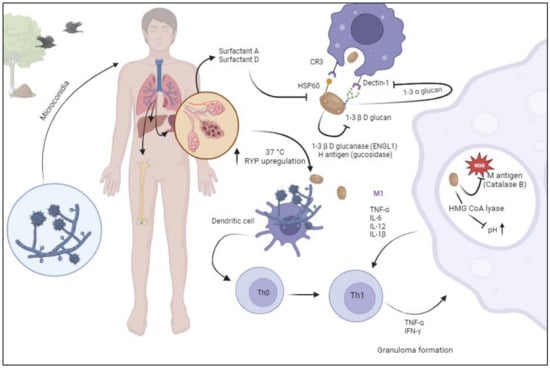
3. Epidemiology and Risk Factors
4. Clinical Presentation
| Clinical Presentation | Severity of Disease | Clinical Symptoms | Radiographic Changes | Laboratory Abnormalities |
|---|---|---|---|---|
| Pulmonary (19–36%) | Mild (4–8%) | Fever (90%) | Abnormal chest X ray (50–70%) | Pancytopenia |
| Acute (symptoms less than 1 month) | Moderate (61–63%) | Shortness of breath (60%) | Abnormal CT chest (87–100%) | Transaminitis |
| Subacute (symptoms over 1 month) | Severe-Critical (27–35%) | Cough (39%) | Multiple nodules | Elevated lactate dehydrogenase |
| Chronic (symptoms over 1 month with cavitary lesions) |
Diarrhea (35%) | Miliary nodules (6%) | Elevated inflammatory markers | |
| Progressive Disseminated Histoplasmosis (64–81%) | Fatigue | Bibasilar infiltrates | Hypercalcemia | |
| Pulmonary (79–86%) | Malaise | Adenopathy (25%) | Elevated creatinine | |
| Bone marrow (21–37%) | Diaphoresis | Abnormal CT abdomen | ||
| Liver (18–22%) | Headache | Hepatosplenomegaly (25–60%) | ||
| Spleen (9–21%) | Weight loss | |||
| Gastrointestinal (7–12%) | Fever of unknown origin | |||
| Central nervous system (6–9%) | ||||
| Skin (2–4%) |
5. Diagnostics
| Overall | Pulmonary | Disseminated | CNS | |
|---|---|---|---|---|
| Culture | ||||
| Blood | 27–63% | 49% | 90% | |
| Lung, respiratory | 57–72% | 60–82% | 77% | |
| Pathology, Cytology | ||||
| Lung, respiratory pathology or cytology | 77% | 86% | 74% | |
| Bone Marrow | 71% | - | 71% | |
| Antigen | ||||
| MVD Ag EIA | ||||
| Urine | 85–100% | 73% | 90–100% | |
| Blood | 86% | 50–59% | 89–93% | |
| BAL | 83% | |||
| CSF | - | - | 78–98% * | |
| IMMY Clarus (urine) | 58% | 72–91% | ||
| MVD LFA | 93% | 50% | 90–96% | |
| Serology | ||||
| ID and/or CF | 33–36% | 62–90% | 28–71% | 51% |
| MVD IgG/IgM EIA | 52% ** | 82–98% * |
5.1. Culture, Histopathology and Cytopathology:
Isolation of H. capsulatum from clinical specimens remains the gold standard for diagnosis of histoplasmosis, though histopathologic or direct microscopic identification are also considered definitive diagnosis [56]. However, having a trained pathologist/microbiologist is imperative to have accurate reports.
Fungemia is present in 63% of patients and is higher in patients with disseminated compared to mild-to-moderate disease (90% vs 49%). Lung or respiratory cultures were positive in 72% of the patients and was more common in disseminated compared to mild-to-moderate disease (77% vs 60%) [13]. Direct visualization of organisms in the bone marrow occurred in 71% of the patients [13].
5.2. Antigen
Detection of H. capsulatum polysaccharides in urine and serum was first developed in 1986 as a sandwich radioimmunoassay. Since then, it has been improved to a second-generation EIA, which allowed for semiquantitative results and reduced the number of false-positive results caused by human anti-rabbit antibodies, and subsequently a third-generation quantitative test with improved sensitivity (MVista Histoplasma galactomannan EIA, USA) [57].
In a study of 18 solid organ transplant recipients with proven histoplasmosis from two university medical centers in the Midwest, antigenuria was present in all the cases [42]. Another report from an endemic area, showed that 12/14 (85%) of the SOT patients with disseminated disease had a positive urine antigen test [41]. The lower sensitivity in this study may be related to the use of older generation test for antigen detection as the study spanned from 1997 to 2007.
In a large multicenter study involving 152 SOTs (kidney: 51%, liver: 16%, kidney/pancreas: 14%, heart: 9%, lung: 5%, pancreas: 2%, others: 2%) with histoplasmosis, Assi et al., showed that urinary antigen testing was the most sensitive test, being positive in 93% of all patients [13]. Antigenemia was present in 86%. However, the test performed differently depending on the burden of disease. Antigenuria was present in 73% of the patients with pulmonary disease vs 97% of those with progressive disseminated disease (p=0.01). In addition, antigenemia was present in 59% of the patients with pulmonary disease vs 89% of those with progressive disseminated disease (p=0.03) [13].
While the MVista assays are laboratory developed tests requiring processing in a central laboratory, an in vitro urine Histoplasma galactomannan EIA by IMMY (IMMY Alpha EIA) was FDA approved in 2007 and has the advantage of being available for use at local facilities. This is particularly useful in settings outside the United States. The IMMY alpha EIA has a sensitivity of 67% for progressive disseminated histoplasmosis in patients living with HIV [58]. Subsequently, IMMY developed a new platform using an analyte-specific reagent H. capsulatum antigen EIA (Clarus IMMY Histoplasma GM EIA, IMMY, USA). This in vitro test has been FDA cleared [59]. In their initial reports, the Clarus IMMY had a sensitivity of 64.5%. with a cutoff of > 0.5 ng/mL which increased to 80.7% if the cutoff was lowered to >0.15 ng/mL [60]. The specificity at those cutoff levels was 99.8% and 96.3%, respectively. In a reevaluation of those cutoff levels, Theel et al. showed an improved positive and negative agreement with MVD EIA (82.3%). Of note, all indeterminates result were removed from the analysis [61]. A subsequent study from clinical samples, showed it had a lower overall sensitivity compared to MVista EIA (72% vs 96%) [62]. Of note, both tests detected all patients with progressive disseminated histoplasmosis. A more recent study in patients living with HIV/AIDS with progressive disseminated disease showed that the sensitivity of Clarus IMMY EIA was 91.3% with a specificity of 90.9% [59]. It is unclear if the differences observed in the prior studies are related to study populations (immunocompetent vs immunocompromised) and disease burden (pulmonary histoplasmosis vs progressive disseminated histoplasmosis).
The detection of Histoplasma galactomannan in other body fluids including bronchoalveolar lavage and cerebrospinal fluid has been described for the diagnosis of pulmonary and central nervous system histoplasmosis, respectively. Of note the galactomannan tested in body fluids of patients with histoplasmosis is identical to galactomannan detected in blastomycosis [63]. Histoplasma galactomannan was detected in 84% of cases of pulmonary histoplasmosis and 83.3% of patients with pulmonary blastomycosis. The test had a specificity of 98% in patients with other pulmonary infections [64]. The sensitivity of the test in CSF for central nervous system histoplasmosis was 78% with a specificity of 97% [65].
Recently, a Histoplasma galactomannan antigen qualitative lateral flow assay was developed (MVD Histoplasma LFA). The test can be read manually and by an automated reader. It has an overall sensitivity of 78.8% with a specificity of 99.3%. While positive in only 50% of patients with pulmonary histoplasmosis, it was positive in 91.3% with disseminated histoplasmosis [66]. Furthermore, its sensitivity in immunocompromised patients was 93.6%.
In a study of patients living with HIV and progressive disseminated histoplasmosis in Mexico, the sensitivity of the MV LFA was 90.4%, which was similar to Clarus IMMY EIA (91.3%) [59]
In a study by Caceres et al. in Colombian patients living with HIV, the Histoplasma LFA showed an excellent analytical performance with a sensitivity of 96% and specificity of 90% [67,68].
OptimumIDX recently developed another lateral flow platform (OIDx Histoplasma LFA). The test had a sensitivity of 92% but a specificity of 32% [69]. In a study from Ghana, the OIDx Histoplasma LFA had a positive concordance with Clarus IMMY of 98.4%, though negative concordance was not reported [70].
The galactomannan detected in the MiraVista antigen assay has almost the same molecular characteristics as those in Blastomyces dermatidis, Paracoccidioides braziliensis and Talaromyces marnefii [71]. Hence, they express almost complete cross-reactivity. Low-level cross-reactivity occurs with the galactomannan detected in coccidioidomycosis and other dimorphic fungi [71-77].
5.3. 1-3-β-D-glucan
1-3 β-D-glucan is a component of the cell wall of most fungi and its detection in serum has been used to identify patients with invasive fungal diseases [78]. Previous studies have shown that serum 1-3-β-D-glucan is present in over 85% of all patients with histoplasmosis [79,80]. However, the lack of specificity and high false-positive rate in patients without an invasive fungal infection limits its use as a diagnostic tool for histoplasmosis.
5.4. Aspergillus galactomannan
The test for the detection of Aspergillus galactomannan (Platelia, Bio-Rad) is widely used to diagnose invasive aspergillosis [81]. Several studies have shown that 67%-100% of the patients with disseminated histoplasmosis have positive Aspergillus galactomannan test [82-84]. In an experimental model of invasive pulmonary aspergillosis, specimens that were positive for Aspergillus galactomannan (including those with very high levels) were negative for Histoplasma antigen EIA [84]. The cross-reactivity has also been noted with paracoccidioidomycosis (50%) and cryptococcosis (50%) [82].
Of note, patients with aspergillosis do not have positive urine or serum Histoplasma antigen EIA [84]. For that reason, it is important to rule out histoplasmosis and other fungi in the setting of a positive Aspergillus galactomannan.
5.6. Serology
The most common tests assessing antibodies against histoplasmosis include complement fixation (CF), immunodiffusion (ID) and ELISA immunoassay (EIA). CF detects antibodies against two antigens: histoplasmin and a yeast antigen. Historically, titers of 1:32 or above are suggestive of active histoplasmosis while titers of 1:8 and 1:16 are considered presumptive evidence of histoplasmosis. A four-fold increase is suggestive of progression of the disease [85].
CF fixation or ID were positive in 33% to 36% of all cases but detection of antibody by CF was the basis for diagnosis in only one of 142 cases in the Assi report [13,41]. While 62% of the pulmonary cases were positive, only 28% of disseminated cases were positive [13].
The ID test detects the presence of antibodies against antigens M and H. The M band is detectable in most immunocompetent patients with histoplasmosis (80%) but persists over time and does not distinguish between prior exposures or active disease. The presence of antibodies against H antigen detects active infection, though it lacks sensitivity (20%) [86].
ID and CF are complex and less standardized than EIA and not widely available in clinical laboratories. Prior reports indicate that antibodies by ID and CF are positive in 36-70% of the cases [13,73]. In those reports, the authors do not provide the individual sensitivity of each test. However, data in solid organ transplant recipients are lacking. In patients with solid organ transplantation, antibody detection by EIA was positive in 50% of the patients (unpublished data).
Combining the detection of antigen and antibodies by EIA increased the sensitivity for the diagnosis of pulmonary histoplasmosis from 67% for antigen alone and 89% for antibody alone to 96.3%[87]. However, this cohort did not include patients with solid organ transplantation. The combination of detecting antigen and antibodies by EIA has also been studied in cerebrospinal fluid of patients with central nervous system histoplasmosis. In that study, Bloch et al. showed that the sensitivity of the test increased from 78% for antigen alone and 82% for antibody alone to 98% [88].
Of not, antibodies are detectable only 4-8 weeks after the initial infection and has lower sensitivity in the immunocompromised hosts [86].
5.7. Molecular tests
Currently, there are no FDA approved assays to detect histoplasmosis from human samples. However, a chemiluminescent DNA probe (AccuProbe test; Gen-Probe Incorporated 2011) is commercially available which decreases the time to identify the pathogen growing in culture [89]. A proteome-based technique, matrix-assisted laser desorption ionization-time of light mass spectrometry (MALDI-TOF MS), has been shown to be highly accurate for the identification of H. capsulatum from cultures. This test can identify yeast forms and early mycelial cultures [90]. Of note, MALDI-TOF is only useful for the identification of Histoplasmosis in instruments with Vitek MS v3.0 database but not on MALDI Biotyper, Bruker Daltonics. The latter was unable to identify dimorphic fungi due to the lack of the fungal reference spectrum in its database [91].
Multiple laboratory-developed Histoplasma-specific PCR assays though the clinical validation has not been described. Broad-range PCR of fungal 28S ribosome and internal transcribed spacers (ITS) have been used for the isolation of histoplasmosis in different tissues (e.g., paraffin embedded biopsies) [92]. In recent years, metagenomics next-generation sequencing has been used to the identification of rare infections [93]. Recently, this assay was able to correctly identify 2 different cases of disseminated histoplasmosis [94].
- Management
While most immunocompetent patients do not require therapy, all solid organ transplant recipients with histoplasmosis require therapy [47]. The agent of choice and duration of therapy depends on the severity of the clinical presentation.
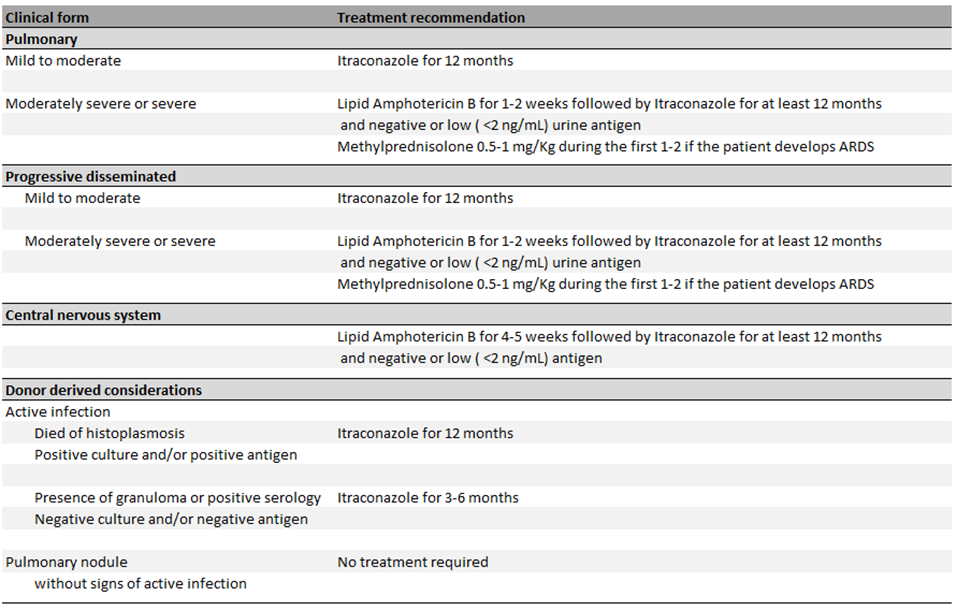
Most of the data has been extrapolated from trials in patients living with HIV. In patients presenting with disseminated disease, amphotericin B is associated with an increased clearance of cultures and more rapid decline on median antigen levels in serum [95]. Liposomal amphotericin B is associated with less toxicity and mortality and is considered the agent of choice for induction therapy in patients with severe disease [96]. The American Society of Transplantation Infectious Diseases Community of Practice recommends continuation of the induction therapy for a minimum of 1 to 2 weeks with clinical evidence of improvement [37]. In patients with CNS involvement, longer courses of induction therapy are recommended (i.e., 4 to 6 weeks) [37,97]. Following completion of induction therapy, therapy should step-down to oral itraconazole. While the concomitant use of itraconazole and amphotericin B has not been associated with any antagonistic effects, in animal models fluconazole has been shown to antagonize amphotericin’s reduction of fungal burden [98]. Itraconazole is preferred over fluconazole due to concerns of lower efficacy and development of resistance [98]. Other triazoles, including voriconazole, posaconazole and isavuconazole have been studied. Voriconazole has been shown to have a low barrier for resistance while posaconazole remained active in strains with fluconazole resistance [99]. Furthermore, voriconazole has been associated with increased mortality in the first 42 days when compared to itraconazole [100]. Isavuconazole also displayed a higher barrier to resistance and may be effective as step-down oral therapy for histoplasmosis [101]. Patients with mild to moderate infection may be treated with itraconazole monotherapy [102].
Conventional itraconazole (referred to here as itraconazole), is only available in oral formulations (i.e., capsules and solution). It has a suboptimal absorption with variable pharmacokinetics [99]. Absorption of oral capsules can be improved by consumption with acidic drinks (e.g., sodas) and fatty meals. Conversely, the oral solution is better absorbed on an empty stomach and is not affected by acid-suppressive therapies. Given the variable pharmacokinetic, therapeutic drug monitoring is recommended in all patients [100]. The metabolism of itraconazole produces an extensive number of metabolites, of those, hydroxyitraconazole has been found to have antifungal activity which is comparable to the parent drug [99]. When measuring serum concentrations by chromatographic tests (High-performance liquid chromatography [HPLC] or liquid chromatography mass spectrometry [LC-MS]) the sum of itraconazole and hydroxyitraconazole provide the total bioactive drug concentration.
The therapeutic target level of itraconazole/hydroxyitraconazole for the treatment of histoplasmosis is unknown and the current recommendations of maintaining levels of at least 1.0 mcg/mL are derived from extrapolation of other fungal infections [100-102]. In patients with oropharyngeal candidiasis, itraconazole serum concentrations <1.0 mcg/mL were associated with treatment failures [103]. A recent study has shown that subtherapeutic concentrations (<1.0 mcg/mL) of itraconazole and hydroxyitraconazole were associated with increased mortality in patients with blastomycosis [104]. It is unclear if a concentration above 1.0 mcg/mL of the parent drug (itraconazole) or a combination of parent drug + metabolite (Itraconazole/hydroxyitraconazole) is required for improved outcomes in the management of histoplasmosis.
A newer formulation of itraconazole labeled super bioavailable (SUBA)-itraconazole has been developed with less variability under fasted condition [105]. However, its use has been limited due to its increased cost.
It is important to assess drug interactions as triazoles are strong inhibitors of the cytochrome P450 isoenzymes, particularly CYP3A4. Careful adjustment and drug level monitoring of other medications, including calcineurin inhibitors is pivotal, as it could lead to supratherapeutic levels [100].
Immune reconstitution syndrome has been observed in solid organ transplant recipients during therapy of Histoplasmosis. Withdrawal of immunosuppression and recovery of CD4+ T cell and CD8+ lymphocytes leading to a proinflammatory state. The management of immune reconstitution syndrome is predominately supportive, though in cases with severe inflammation, corticosteroids can be considered [106,107].
The 2007 Infectious Disease Society of America Histoplasmosis Treatment Guidelines and the American Society of Transplantation Infectious Diseases Community of Practice recommend that therapy should be continued for a minimum of 12 months [97,101]. Assi et al. showed that relapses occur in up to 6% of the patients with two thirds occurring in the first 2 years following the diagnosis. Of the patients who relapsed, 50% were treated with less than 7 months of therapy. The only risk factor associated with relapse was failure to reduce calcineurin inhibitor dosage [13].
The 2007 Infectious Disease Society of America Histoplasmosis Treatment Guidelines and the American Society of Transplantation Infectious Diseases Community of Practice recommend monitoring urine and serum Histoplasma antigen levels measured by EIA at the time treatment is initiated, at 2 weeks, 1 month, then every 3 months during therapy and up to 6 months following completion of therapy [97,101]. Persistent low-level antigenuria is not infrequent though patients with a urine Histoplasma antigen EIA level of >2 ng/mL at time of stopping therapy are more likely to relapse [13]. The current guidelines recommend continuation of therapy until urine Histoplasma antigen EIA level of <2 ng/mL [97,101].
- Novel antifungal therapies and strategies
Recently, the World Health Organization has endorsed a single high-dose regimen of amphotericin B for the treatment of cryptococcal meningitis [108,109]. This regimen is not only associated with fewer side effects, but it also has the advantage of limiting hospitalization costs. A recent prospective randomized multicenter open-label trial comparing a 1- or 2 dose induction therapy with liposomal amphotericin B versus standard of care (daily liposomal amphotericin B for 2 weeks) has been reported [110]. The authors used a single dose (10 mg/Kg of LAmB) or two doses (10 mg/Kg of LAmB on D1 and 5 mg/Kg of LAmB on day 3) followed by step—down therapy with itraconazole. The authors concluded that one day induction therapy with 10 mg/Kg of LAmB was safe and was not inferior to standard of care. However, this regimen has not been tested in solid organ transplant recipients and further data from the phase 3 study is warranted before this regimen could be extrapolated to other populations (clinical trial number 05814432).
A new oral formulation of amphotericin B (oral lipid nanocrystal amphotericin B) has been developed. In a recent randomized control trial, Boulware et al. evaluated the antifungal efficacy of oral lipid nanocrystal amphotericin B with flucytosine vs standard of care for the treatment of cryptococcal meningitis. They found that the newer oral formulation demonstrated similar antifungal activity, similar survival and was associated with fewer adverse side effects compared to standard of care [111]. There are no trials assessing its activity for treatment of histoplasmosis.
- Peri-transplant donor and recipient considerations
Donor-derived histoplasmosis is estimated to occur in approximately 1:10,000 transplants, though this incidence varies according to the endemicity of the area. In a large study of 449 patients in a hyperendemic area that underwent solid organ transplantation, 24% of the recipients were seropositive (M precipitins band or CF titer ≥1:8) though there were no post-transplant infections noted at 16 month follow up. The authors concluded that the risk of reactivation is low and that routine prophylaxis with antifungal agents for those with radiographic or serological evidence of a prior infection (over 2 years) is not indicated [6]. The current guidelines state that active histoplasmosis within 2 years from transplantation may warrant prophylaxis, but did not specify the duration of prophylaxis [112].
A recent report summarized the cases of donor-derived endemic mycoses including seven cases of donor derived-histoplasmosis [113]. None of the donors were known or suspected to have histoplasmosis at the time of donation. In donors in whom there are concerns for active infection (e.g. granulomatous disease or history of histoplasmosis), cultures of the allograft, Histoplasma antigen and antibody results should be measured before the organs are procured. If the donor has evidence of active disease Histoplasma antigen positive in urine or serum or Histoplasma antibody positive by EIA, transplantation should be delayed until there is evidence that the donor does not have active histoplasmosis.
If there is evidence of histoplasmosis in the explanted organ (e.g. positive fungal stains or presence of granulomas), graft cultures and serologies should be performed. If the cultures are negative but the serologies show an H and/or M precipitin band, or CF ≥1:32 then the recipient should receive prophylaxis with itraconazole for 3-6 months. If there is only evidence of granuloma and the CF is between 1:8 and 1:16, then urine and serum antigen monitoring every 3 months for 1 year is reasonable. If donor died due to histoplasmosis (recognized after transplantation has occurred, as otherwise the donation is not advised) or if the cultures or antigen are positive, then the recipient should be treated for at least 1 year [112].
- Conclusions
Histoplasma capsulatum, is the etiological agent for histoplasmosis. It is a dimorphic fungus that grows as a mold in the environment and as a yeast in human tissues. It has a broad global distribution with shifting epidemiology likely due to climate change and anthropomorphic activity. Infection in immunocompetent recipients is usually asymptomatic or with mild respiratory symptoms, though severe forms including progressive disseminated disease can occur. Solid organ transplant recipients are at increased risk for developing symptomatic disease with progressive disseminated histoplasmosis. Antigen detection in bodily fluids remains the most sensitive test for diagnosis. Most data on treatment recommendations has been derived from other populations, including advanced HIV. Treatment is indicated in all solid organ transplant recipients for at least 12 months.
More research is required to test efficacy of the current treatment recommendations in solid organ transplant recipients and to develop less toxic treatment regimens with less drug interactions. Furthermore, further research is needed to tailor the length of therapy in immunocompromised host.
This entry is adapted from the peer-reviewed paper 10.3390/jof10020124
