Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cancer surgery is an essential treatment strategy but can disrupt patients’ physical and psychological health. Perioperative medicine is a growing speciality that aims to improve clinical outcome by preparing patients for the stress associated with surgery. Preparation should begin at contemplation of surgery, with universal screening for established risk factors, physical fitness, nutritional status, psychological health, and, where applicable, frailty and cognitive function.
- cancer surgery
- prehabilitation
- perioperative medicine
1. Introduction
Cancer treatment heavily relies on surgery, encompassing preventative, diagnostic, curative, palliative, and reconstructive interventions [1]. However, these procedures, while essential, introduce considerable trauma and physiological disruption, posing substantial risks to patients’ physical and psychological well-being. Despite advancements such as enhanced recovery programs, minimally invasive surgical techniques, and robotic surgery, elective cancer surgery remains associated with a notable mortality risk [2]. The prevalent focus on traditional short-term reporting potentially obscures the full scale of the issue, given the consideration of ‘late mortality’ occurring between days 31 and 90, or even later [3]. Estimates of postoperative morbidity vary based on factors such as heterogenous outcome reporting, level of hospital infrastructure [4], surgical site, and complexity [5]; however, the considerable impacts on patients, families, and global healthcare systems are widely acknowledged [2][5][6]. Urgent and elective cancer surgeries exhibit similarly unfavourable outcomes [7]. Notably, over half of patients aged 60 and above who undergo major abdominal surgery fail to regain their preoperative functional capacity, quality of life, or physical fitness [8][9]. Perioperative risk is multi-factorial, a function of preoperative condition of the patient, surgical complexity, and anaesthetic administration. Cancer patients face particular burden due to the deconditioning nature of disease, and neoadjuvant treatment and potential for multiple exposures to anaesthetics during diagnostic and treatment phases [10].
A patient’s preparedness for surgery’s physiological and psychological impact is not guaranteed. Historically, healthcare systems have prioritized the operation and disease itself. While historically healthcare prioritised the operation and disease, a compelling case supports postoperative outcomes being primarily influenced by patient resilience, i.e., their ability to counteract perioperative stressors [11]. This paradigm shift positions the surgical response as the primary ‘disease process,’ urging a recalibration of perioperative care to centre on optimising patient resilience at the time of contemplating surgery. Perioperative medicine now encompasses comprehensive support from initial suspicion of diagnosis to full recovery [12]. The interval between diagnosis and surgery presents an opportunity to tailor care for the changing patient demographic with intricate health requirements. Achieving this entails meticulous comorbidity management, arranging suitable enhanced care facilities, supporting health-enriching behaviours, and fostering informed discussions regarding the appropriateness of surgery, particularly when potential harm might outweigh the benefits. Regrettably, the prevalent care models rarely align with these risk-focused goals, often prioritizing siloed health system concerns such as treatment timeline, clinic availability, operating room capacity, and postoperative care resources. Reconfiguring surgical processes to facilitate patient-centric pathways, rooted in comprehensive risk assessment, can yield manifold advantages [13]. At this critical juncture, facing escalating cancer care demands and limited resources, adopting a business process re-engineering approach to perioperative medicine aligns with the widely-adopted “Quintuple Aim” of healthcare, i.e., enhancing care experiences, bolstering population health, reducing per capita healthcare, addressing clinician burnout, and advancing health equity [14][15][16].
2. Functional Capacity: Navigating Definitional Controversies of Perioperative Risk Assessment
Traditional preoperative risk assessment, focused on surgical complexity, coexisting medical conditions, and more recently incorporating an evaluation of functional capacity, often relies on subjective judgments of physical fitness [17]. However, resuming daily activities after surgery necessitates an integrated physiological response, involving cardiopulmonary, neuromuscular, musculoskeletal, metabolic, and psychological systems. Contemporary evidence advocates for a broader risk definition, extending to proficiencies essential for meaningful postoperative function, including physical fitness, nutritional status, psychological well-being, cognitive function, and frailty [18][19][20]. Poor dietary intake, nutritional status and sedentarism can induce inflammatory, metabolic, and endocrine processes that promote cancer development through accumulated DNA damage, diminished cancer apoptosis, and sustained proliferative signalling [21][22]. A sensitive screening process should identify at-risk individuals for comprehensive assessment by a proficient clinician within a healthcare facility. Given evolving evidence, achieving accurate and scalable risk assessment requires a comprehensive reorganization of perioperative medicine services [13].
In perioperative medicine, the concept of functional capacity is evolving from a single-dimensional to a multi-faceted evaluation, requiring further research on measurement methodologies, component weighting, and feasibility for widespread implementation. Balancing comprehensiveness, practicality, and sensitivity to local context is crucial in defining and utilizing functional capacity assessments for improved perioperative care.
2.1. Physical Fitness
Empirical evidence from the 1990s linked objectively measured low physical fitness with heightened postoperative risk in elderly patients undergoing major intra-cavity surgery [23]. Recent systematic reviews and meta-analyses have affirmed these findings across diverse cancer surgical populations and procedures [24][25][26]. The well-established link between sedentarism and cancer development further promotes the imperative to adequately establish preoperative physical fitness. Moreover, neoadjuvant treatment (NAT) is integral to preoperative cancer care, aiming to enhance circumferential margins. However, NAT introduces cardiovascular deconditioning through combined effects of direct cardiotoxicity, unmasking of compensatory mechanisms of cardiac dysfunction, and mitochondrial degradation [24][27][28].
In the perioperative domain, physical fitness assessment often relies on subjective clinician estimates using the American Society of Anesthesiologists (ASA)’s Physical Status Classification System or metabolic equivalent of tasks (METs), determining fitness to proceed if the patient exceeds four METs without symptoms [29]. Moreover, there is significant discordance between clinician-assessed and patient-reported exercise capacity [30]. Such techniques inaccurately gauge patient fitness, limiting predictive utility [26][31], and have prompted calls for systematic and objective screening at contemplation for surgery [11][12].
2.2. Nutritional Status
Malnutrition, an imbalance between nutrient/energy intake and requirements, leads to reduced metabolic reserve, sarcopenia, cachexia, and compromised physical fitness [32][33]. Strong associations exist between weight loss, low muscle mass, and reduced survival in various cancers, with patients experiencing these issues surviving about 8 months compared to 28 months for those without [34][35]. Cancer cachexia, affecting 50–80% of cancer patients, arises from tumour-induced anorexia, catabolic effects, altered nutrient metabolism, gastrointestinal tract obstruction, and reduced food intake [36]. Inadequate dietary intake due to pain, anxiety, and depression deserves attention.
The latest guidelines from the European Society for Clinical Nutrition and Metabolism (ESPEN) underscore the link between nutritional status and postoperative outcomes, with malnourished surgical patients experiencing elevated morbidity, mortality, prolonged hospital stay, unplanned readmission rates, and increased inpatient care costs [37][38]. Loss of skeletal muscle mass and function (sarcopenia) is associated with reduced overall survival and increased risk of postoperative complications, across a range of cancer types [34][39]. Patients facing gastrointestinal and head and neck surgery face the highest risk of malnourishment due to the cancer process, effects of systemic anti-cancer treatment and malabsorptive states [39][40]. Severe malnutrition was found in 33% of patients undergoing elective gastric or colorectal cancer surgery, with a positive association with 30-day mortality [40], while preoperative nutritional risk doubles the chance of 30-day readmission [41]. Addressing malnutrition-mediated surgical risk before surgery, even if causing a brief delay, supports a proactive shift to nutritional screening, targeted assessment, and personalized intervention.
2.3. Psychological Health
The psychological impact of receiving a cancer diagnosis and facing surgery should not be underestimated [19]. Cancer diagnosis and surgery have a substantial psychological impact, with around 50% experiencing clinically significant distress across various cancer types [42][43]. A meta-analysis reported mood disorders in 30–40% of hospital inpatients, with no significant differences between palliative and non-palliative settings [43]. Pooled data from 16 prospective studies, reporting 4353 cancer related deaths, revealed that higher levels of distress were associated with a 32% greater mortality risk [44].
Depression and anxiety are associated with poorer outcomes after cancer surgery, including pain [45], delayed wound healing [46], and increased length of hospital stay [47]. High depression and low self-efficacy to self-manage health conditions at diagnosis is predictive of lower quality of life, and can impact treatment option decision-making and reduce mental health for up to two years after surgery [48][49].
A systematic review of 16 studies by Mavros et al. reported heterogenous measures of distress and postoperative outcome; however, there was consistent association between the presence of one or more components of psychological distress and poorer early postoperative outcomes, up to 30-days following surgery [46]. A review of 13 studies by Rosenberger et al. reported that mood, anxiety, and depression predicted short term postoperative outcome, length of hospital stay, self-reported recovery, and long-term pain [50]. Importantly, both of these systematic reviews [46][50] reported association between positive psychological traits and improved recovery. Self-efficacy, low pain expectation, external locus of control, optimism, religious faith, and anger control were associated with favourable postoperative outcomes, suggesting that altering the psychological well-being of people with cancer prior to surgery may have the potential to promote better recovery [19] and improve ongoing compliance with treatment.
2.4. Frailty
Frailty is a widely used term describing a cumulative, multi-factorial health decline that heightens vulnerability to further deterioration after a stressor event [51][52]. Often viewed as age-related decline, frailty is more accurately a biological expression shaped by genetic and environmental factors, influenced by lifestyle choices like physical activity, diet, smoking, and alcohol use [51]. Among the frail, acute medical events lead to disproportionate changes in health status, with cancer patients particularly susceptible to the cumulative psycho-physiological insults asserted by oncogenesis, anti-cancer treatment, malabsorptive nutritional states, and disease-related symptoms [53]. Characterised by weakness, fatigue, decreased mobility, and cognitive impairment, frail patients have increased vulnerability to falls, delirium, disability, institutionalisation, morbidity, and death [51][54]. Both frailty and cancer incidence rise with age, highlighting the relevance of frailty to cancer surgery services amid a rapidly aging population and an increasing age of people with cancer undergoing surgery [55].
Frailty increases the risk of poor postoperative outcomes, even following minor procedures [20][56][57]. In a study of over 23,000 patients undergoing non-cardiac surgery, frailty was linked to a 1.5-fold increase in postoperative healthcare cost [58]. A meta-analysis of 45,000 frail patients undergoing non-cardiac surgery revealed mortality rates of 1.55% following the lowest-stress surgery (e.g., cystoscopy). The rate of 180-day mortality reached 43% for very frail patients undergoing moderate-stress procedures (e.g., laparoscopic cholecystectomy) [59]. Despite this robust association, frailty is inadequately considered during perioperative assessment and subsequent shared decision-making, risking patients undergoing inappropriate procedures or being excluded from potentially beneficial ones that could be supported by interventions that modify frailty characteristics [60][61][62].

2.5. Cognitve Function
While cognitive decline is a normal process of aging, the rate and underlying aetiology are highly heterogenous. Severity lies on a continuum with three widely accepted phases. Pre-clinical or Subjective Cognitive Decline (SCD) is characterised by self-reported cognitive decline without measurable symptoms [63][64]. Mild cognitive impairment (MCI) is diagnosed with cognitive changes, abnormal function, and no dementia [65]. MCI sufferers can be sub-grouped as amnestic or non-amnestic MCI, with implications for understanding and completing postoperative recovery plans. Finally, dementia is the most severe stage, with deficits across multiple cognitive and functional domains [66]. Progression is not inevitable, and while SCD and MCI phases may have slow progression, a recent review noted accelerated neurocognitive decline in cancer patients over 65, making perioperative clinicians potential early identifiers [66][67].
Global dementia cases are projected to triple to 130–175 million by 2050, with significant regional variations [68].
Each stage of cognitive impairment increases healthcare utilization, institutionalization, and mortality. Preoperative cognitive impairment predicts delirium, postoperative complications, 12-month mortality, 30-day readmission, discharge to assisted care, and long-term neurocognitive issues [69]. Postoperative cognitive decline accelerates in elderly patients with pre-existing cognitive impairment [70]. A 2018 state of the science summit coined the overarching term Perioperative Neurocognitive Disorder (PND) to capture the range of associated conditions [71].
Evidence suggests modifiable factors like smoking, alcohol consumption, obesity, physical inactivity, and poorly controlled diabetes contribute to cognitive decline [72]. While perioperative interventions may have limited impact, a cancer diagnosis and interactions with healthcare professionals offer opportunities for lifestyle modifications with potential long-term cognitive health benefits.
2.6. Prehabiltation
Definition: Initially focusing on exercise, prehabilitation has rapidly evolved into a multimodal model that encompasses exercise, nutrition, psychological support, smoking cessation, and alcohol use moderation, underpinned by supported behaviour change, to address the modifiable components of functional capacity and frailty [73].
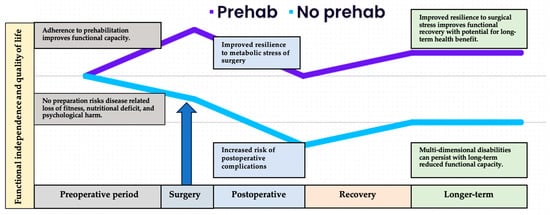
History: Cancer survivorship, integral to gold-standard, person-centred care, spans from diagnosis to end of life. In 2006, a pivotal report by the Institute of Medicine and the National Research Council emphasized the need for focused attention on the “period following first diagnosis and treatment” [74]. While traditional perioperative rehabilitation reacted to treatment-induced impairment, widely adopted enhanced recovery after surgery (ERAS) programs adopted a proactive stance. These programs, grounded in early postoperative reintroduction of physical activity and nutrition, aimed to prevent surgery-associated morbidity, and facilitate early hospital discharge [75]. Building on this progressive model of care, prehabilitation is a multidisciplinary approach that aims to optimise a person’s physical and emotional resilience in preparation for the upcoming surgical procedure (see Figure 1).

Figure 1. Overview of the differential health trajectories related to adherence to prehabilitation prior to surgery. The cancer care continuum begins at the point of diagnosis and contemplation of surgery. While the health system works through necessary diagnostic and administrative processes, the patient can begin to prepare to face the metabolic stress of surgery. Those who arrive at the operating theatre physically fit, nutritionally replete, and psychologically prepared are likely to suffer less severe response to surgical stress, recover more quickly and more fully, and regain previous or improved functional capacity and quality of life in the longer-term postoperative period.
Evidence: Existing research offers promising yet inconclusive evidence of the impact on cancer surgery outcomes. While certain studies suggest a shorter hospital stay (LOS) and a reduced occurrence of postoperative complications [76][77][78], others report non-significant effects on these outcomes [76][79]. Emerging evidence suggests that exercise during neoadjuvant therapy (NAT) not only mitigates associated mitochondrial degradation [80], but may improve treatment tolerance, reduce toxicity, and augment tumour regression [81]. This finding gains biological plausibility from murine models, making it appealing and deserving of substantial investment in well-conducted controlled research trials [82].
Key principles: Despite acknowledging the need for cautious interpretation of the evidence, calls have grown for widespread adoption of surgical prehabilitation [83][84] and a range of foundation principles have emerged to address the issues associated with preoperative risk.
-
Detailed mapping of patient pathways and establishment of efficient clinical framework to support large throughput of numbers in the time limited gap between diagnosis and surgery. Figure 2 illustrates a proposed evidence-based framework for preoperative management that incorporates prehabilitation [73].
-
Multimodal prehabilitation programmes which incorporate the foundational pillars of prehabilitation, physical fitness, nutrition, and psychological support are likely to be the most effective. Patients may require a tailored focus on individual elements based upon screening and assessment of need [32].
-
The multidisciplinary team is required to support multimodal prehabilitation, with specialist assessment and individualised prescription [32]. Complexity of the intervention means clinicians interested in developing prehabilitation services should identify and engage with key stakeholders, including funders, as early as possible in the development process [85][86].
-
Recognition of challenges faced by patients in making lifestyle changes when faced with the effects of cancer diagnosis and upcoming treatment. Appointment-based, local, and supervised facilities can improve adherence to prehabilitation routines [88].
-
Exercise prescriptions should adhere to international guidance, aiming for 150 min of moderate intensity aerobic exercise per week, or 75 min of vigorous intensity aerobic exercise per week. Given the time pressed nature of the preoperative period, high-intensity interval training (HIIT) sessions are a safe, effective, and time-efficient method of improving physical fitness [89]. Patients should also complete two sessions of strengthening exercises per week. Patients with pre-frailty may benefit from additional balance and strength training [90].
-
Improving nutritional status supports increased physical activity and exercise, and may halt or correct cancer cachexia and improve body composition [85][91]. There is little evidence to support universal dietetic counselling; however, signposting to healthy-eating resources is recommended by prehabilitation guidance [83]. Patients identified at intermediate risk through unintended weight loss, moderate weight loss, and/or unfavourable body composition, as well as those increasing their physical activity and exercise, may also benefit from targeted dietetic counselling and/or oral nutritional supplementation [92], supervised by qualified dietetic professionals [91]. This does assume a functioning gastrointestinal tract. Where oral nutrition and supplementation does not meet elevated metabolic demands, enteral supplementation would be preferred over the parenteral route, which should only be delivered under professional prescription in a specialist inpatient setting [91].
-
Psychological prehabilitation is less well studied; however, patients with cancer who have anxiety and depression should receive targeted behavioural techniques such as relaxation, counselling, and emotion management interventions [93]. Patients with pre-existing and/or severe psychopathology should receive specialist psychological or psychiatric therapies [94].
-
‘Surgery schools’ provide information on what patients can expect before and after surgery, and instruction in self-management of their preparation for surgery through behavioural change. Schools should deliver accepted guidance on increasing physical activity, nutrition, weight management, smoking cessation, and alcohol consumption in line with government guidelines [95].

Figure 2. Overview of the proposed framework for optimal preparation prior to surgery. The period between diagnosis and surgery can be repurposed from waiting time to preparation time. This is reliant upon early screening for risk and targeted assessment, informing meaningful shared decision-making, surgical education, and personalised prehabilitation prescription, underpinned by behaviour change, established medical management, and enhanced recovery techniques.
This entry is adapted from the peer-reviewed paper 10.3390/curroncol31020046
References
- Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; Autier, P.; Aggarwal, A.; Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015, 16, 1193–1224.
- International Surgical Outcomes Study Group TISOS. Global patient outcomes after elective surgery: Prospective cohort study in 27 low-, middle- and high-income countries. Br. J. Anaesth. 2016, 117, 601–609.
- Resio, B.J.; Gonsalves, L.; Canavan, M.; Mueller, L.; Phillips, C.; Sathe, T.; Swett, K.; Boffa, D.J. Where the Other Half Dies: Analysis of Mortalities Occurring More Than 30 Days after Complex Cancer Surgery. Ann. Surg. Oncol. 2021, 28, 1278–1286.
- Meara, J.G.; Greenberg, S.L. The Lancet Commission on Global Surgery Global surgery 2030: Evidence and solutions for achieving health, welfare and economic development. Surgery 2015, 157, 834–835.
- Pearse, R.M.; Moreno, R.P.; Bauer, P.; Pelosi, P.; Metnitz, P.; Spies, C.; Vallet, B.; Vincent, J.L.; Hoeft, A.; Rhodes, A.; et al. Mortality after surgery in Europe: A 7 day cohort study. Lancet 2012, 380, 1059–1065.
- Tevis, S.E.; Kennedy, G.D. Postoperative Complications: Looking Forward to a Safer Future. Clin. Colon. Rectal Surg. 2016, 29, 246–252.
- Mullen, M.G.; Michaels, A.D.; Mehaffey, J.H.; Guidry, C.A.; Turrentine, F.E.; Hedrick, T.L.; Friel, C.M. Risk Associated With Complications and Mortality after Urgent Surgery vs Elective and Emergency Surgery. JAMA Surg. 2017, 152, 768.
- Stabenau, H.F.; Becher, R.D.; Gahbauer, E.A.; Leo-Summers, L.; Allore, H.G.; Gill, T.M. Functional Trajectories before and after Major Surgery in Older Adults. Ann. Surg. 2018, 268, 911–917.
- Lawrence, V.A.; Hazuda, H.P.; Cornell, J.E.; Pederson, T.; Bradshaw, P.T.; Mulrow, C.D.; Page, C.P. Functional independence after major abdominal surgery in the elderly. J. Am. Coll. Surg. 2004, 199, 762–772.
- Walker, E.M.K.; Bell, M.; Cook, T.M.; Grocott, M.P.W.; Moonesinghe, S.R.; Central SNAP-1 Organisation; National Study Groups. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: A cross-sectional observational study. Br. J. Anaesth. 2016, 117, 758–766.
- van Kooten, R.T.; Bahadoer, R.R.; Peeters, K.C.M.J.; Hoeksema, J.H.L.; Steyerberg, E.W.; Hartgrink, H.H.; van de Velde, C.J.H.; Wouters, M.W.J.M.; Tollenaar, R.A.E.M. Preoperative risk factors for major postoperative complications after complex gastrointestinal cancer surgery: A systematic review. Eur. J. Surg. Oncol. 2021, 47, 3049–3058.
- Grocott, M.P.W.; Plumb, J.O.M.; Edwards, M.; Fecher-Jones, I.; Levett, D.Z.H. Re-designing the pathway to surgery: Better care and added value. Perioper. Med. 2017, 6, 9.
- Grocott, M.P. Pathway redesign: Putting patients ahead of professionals. Clin. Med. 2019, 19, 468–472.
- Grocott, M.P.W.; Edwards, M.; Mythen, M.G.; Aronson, S. Peri-operative care pathways: Re-engineering care to achieve the ‘triple aim’. Anaesthesia 2019, 74, 90–99.
- Stiefel, M.; Nolan, K. A Guide to Measuring the Triple Aim: Population Health, Experience of Care, and Per Capita Cost; IHI Innovation Series white paper; IHI—Institute for Healthcare Improvement: Cambridge, MA, USA, 2012.
- Nundy, S.; Cooper, L.A.; Mate, K.S. The Quintuple Aim for Health Care Improvement. JAMA 2022, 327, 521.
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e77–e137.
- Prado, C.M.; Ford, K.L.; Gonzalez, M.C.; Murnane, L.C.; Gillis, C.; Wischmeyer, P.E.; Morrison, C.A.; Lobo, D.N. Nascent to novel methods to evaluate malnutrition and frailty in the surgical patient. J. Parenter. Enter. Nutr. 2023, 47, S54–S68.
- Levett, D.Z.H.; Grimmett, C. Psychological factors, prehabilitation and surgical outcomes: Evidence and future directions. Anaesthesia 2019, 74, 36–42.
- Sandini, M.; Pinotti, E.; Persico, I.; Picone, D.; Bellelli, G.; Gianotti, L. Systematic review and meta-analysis of frailty as a predictor of morbidity and mortality after major abdominal surgery. BJS Open 2017, 1, 128–137.
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816.
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Colorectal Cancer; World Cancer Research Fund: London, UK, 2017.
- Older, P.; Hall, A.; Hader, R. Cardiopulmonary Exercise Testing as a Screening Test for Perioperative Management of Major Surgery in the Elderly. Chest 1999, 116, 355–362.
- Argillander, T.E.; Heil, T.C.; Melis, R.J.F.; van Duijvendijk, P.; Klaase, J.M.; van Munster, B.C. Preoperative physical performance as predictor of postoperative outcomes in patients aged 65 and older scheduled for major abdominal cancer surgery: A systematic review. Eur. J. Surg. Oncol. 2022, 48, 570–581.
- Lee, C.H.A.; Kong, J.C.; Ismail, H.; Riedel, B.; Heriot, A. Systematic Review and Meta-analysis of Objective Assessment of Physical Fitness in Patients Undergoing Colorectal Cancer Surgery. Dis. Colon. Rectum 2018, 61, 400–409.
- Wijeysundera, D.N.; Pearse, R.M.; Shulman, M.A.; Abbott, T.E.F.; Torres, E.; Ambosta, A.; Croal, B.L.; Granton, J.T.; Thorpe, K.E.; Grocott, M.P.W.; et al. Assessment of functional capacity before major non-cardiac surgery: An international, prospective cohort study. Lancet 2018, 391, 2631–2640.
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Adams, V.L.; Bimson, W.E.; Bimson, W.E.; Grocott, M.P.; Jack, S.; Kemp, G.J. The effect of neoadjuvant chemoradiotherapy on whole-body physical fitness and skeletal muscle mitochondrial oxidative phosphorylation in vivo in locally advanced rectal cancer patients—An observational pilot study. PLoS ONE 2014, 9, e111526.
- Peel, A.B.; Thomas, S.M.; Dittus, K.; Jones, L.W.; Lakoski, S.G. Cardiorespiratory Fitness in Breast Cancer Patients: A Call for Normative Values. J. Am. Heart Assoc. 2014, 3, e000432.
- Fan, E.; Cheek, F.; Chlan, L.; Gosselink, R.; Hart, N.; Herridge, M.S.; Hopkins, R.O.; Hough, C.L.; Kress, J.P.; Latronico, N.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Diagnosis of Intensive Care Unit–acquired Weakness in Adults. Am. J. Respir. Crit. Care Med. 2014, 190, 1437–1446.
- Sinclair, R.C.F.; Turley, A.J.; Goodridge, V.; Danjoux, G.R. Does patient reported exercise capacity correlate with anaerobic threshold? Anaesthesia 2009, 64, 459–462.
- Melon, C.C.; Eshtiaghi, P.; Luksun, W.J.; Wijeysundera, D.N. Validated Questionnaire vs Physicians’ Judgment to Estimate Preoperative Exercise Capacity. JAMA Intern. Med. 2014, 174, 1507.
- Carli, F.; Silver, J.K.; Feldman, L.S.; McKee, A.; Gilman, S.; Gillis, C.; Scheede-Bergdahl, C.; Gamsa, A.; Stout, N.; Hirsch, B. Surgical Prehabilitation in Patients with Cancer: State-of-the-Science and Recommendations for Future Research from a Panel of Subject Matter Experts. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 49–64.
- Gillis, C.; Wischmeyer, P.E. Pre-operative nutrition and the elective surgical patient: Why, how and what? Anaesthesia 2019, 74, 27–35.
- GlobalSurg Collaborative and NIHR Global Health Unit on Global Surgery. Impact of malnutrition on early outcomes after cancer surgery: An international, multicentre, prospective cohort study. Lancet Glob. Heal. 2023, 11, e341–e349.
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547.
- von Haehling, S.; Anker, S.D. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 261–263.
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN Guideline ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761.
- Thomas, M.N.; Kufeldt, J.; Kisser, U.; Hornung, H.M.; Hoffmann, J.; Andraschko, M.; Werner, J.; Rittler, P. Effects of malnutrition on complication rates, length of hospital stay, and revenue in elective surgical patients in the G-DRG-system. Nutrition 2016, 32, 249–254.
- Bozzetti, F.; Gianotti, L.; Braga, M.; Di Carlo, V.; Mariani, L. Postoperative complications in gastrointestinal cancer patients: The joint role of the nutritional status and the nutritional support. Clin. Nutr. 2007, 26, 698–709.
- Einarsson, S.; Laurell, G.; Tiblom Ehrsson, Y. Mapping the frequency of malnutrition in patients with head and neck cancer using the GLIM Criteria for the Diagnosis of Malnutrition. Clin. Nutr. ESPEN 2020, 37, 100–106.
- Gillis, C.; Nguyen, T.H.; Liberman, A.S.; Carli, F. Nutrition Adequacy in Enhanced Recovery After Surgery. Nutr. Clin. Pract. 2015, 30, 414–419.
- Mehnert, A.; Hartung, T.J.; Friedrich, M.; Vehling, S.; Brähler, E.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Weis, J. One in two cancer patients is significantly distressed: Prevalence and indicators of distress. Psychooncology 2018, 27, 75–82.
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174.
- Batty, G.D.; Russ, T.C.; Stamatakis, E.; Kivimäki, M. Psychological distress in relation to site specific cancer mortality: Pooling of unpublished data from 16 prospective cohort studies. BMJ 2017, 356, j108.
- Ip, H.Y.V.; Abrishami, A.; Peng, P.W.H.; Wong, J.; Chung, F. Predictors of Postoperative Pain and Analgesic Consumption. Anesthesiology 2009, 111, 657–677.
- Mavros, M.N.; Athanasiou, S.; Gkegkes, I.D.; Polyzos, K.A.; Peppas, G.; Falagas, M.E. Do Psychological Variables Affect Early Surgical Recovery? PLoS ONE 2011, 6, e20306.
- Siddiqui, N.; Dwyer, M.; Stankovich, J.; Peterson, G.; Greenfield, D.; Si, L.; Kinsman, L. Hospital length of stay variation and comorbidity of mental illness: A retrospective study of five common chronic medical conditions. BMC Health Serv. Res. 2018, 18, 498.
- Foster, C.; Haviland, J.; Winter, J.; Grimmett, C.; Chivers Seymour, K.; Batehup, L.; Calman, L.; Corner, J.; Din, A.; Fenlon, D.; et al. Pre-Surgery Depression and Confidence to Manage Problems Predict Recovery Trajectories of Health and Wellbeing in the First Two Years following Colorectal Cancer: Results from the CREW Cohort Study. PLoS ONE 2016, 11, e0155434.
- Nipp, R.D.; El-Jawahri, A.; Fishbein, J.N.; Eusebio, J.; Stagl, J.M.; Gallagher, E.R.; Park, E.R.; Jackson, V.A.; Pirl, W.F.; Greer, J.A.; et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer 2016, 122, 2110–2116.
- Rosenberger, P.H.; Jokl, P.; Ickovics, J. Psychosocial factors and surgical outcomes: An evidence-based literature review. J. Am. Acad. Orthop. Surg. 2006, 14, 397–405.
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762.
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397.
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10.
- Sell, N.M.; Qadan, M.; Silver, J.K. Implications of Preoperative Patient Frailty on Stratified Postoperative Mortality. JAMA Surg. 2020, 155, 669.
- Etzioni, D.A.; Liu, J.H.; O’connell, J.B.; Maggard, M.A.; Ko, C.Y. Elderly Patients in Surgical Workloads: A Population-Based Analysis. Am. Surg. 2003, 69, 961–965.
- Shaw, J.F.; Budiansky, D.; Sharif, F.; McIsaac, D.I. The Association of Frailty with Outcomes after Cancer Surgery: A Systematic Review and Metaanalysis. Ann. Surg. Oncol. 2022, 29, 4690–4704.
- Lin, H.-S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157.
- McGinn, R.; Agung, Y.; Grudzinski, A.L.; Talarico, R.; Hallet, J.; McIsaac, D.I. Attributable perioperative cost of frailty after major, elective non-cardiac surgery: A population-based cohort study. Anesthesiology 2023, 139, 143–152.
- Shinall, M.C.; Arya, S.; Youk, A.; Varley, P.; Shah, R.; Massarweh, N.N.; Shireman, P.K.; Johanning, J.M.; Brown, A.J.; Christie, N.A.; et al. Association of Preoperative Patient Frailty and Operative Stress With Postoperative Mortality. JAMA Surg. 2020, 155, e194620.
- Centre for Perioperative Care. Preoperative Assessment and Optimisation for Adult Surgery including Consideration of COVID-19 and Its Implications; Centre for Perioperative Care: London, UK, 2021.
- Sturgess, J.; Clapp, J.T.; Fleisher, L.A. Shared decision-making in peri-operative medicine: A narrative review. Anaesthesia 2019, 74, 13–19.
- Overview|Shared Decision Making|Guidance|NICE. Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/shared-decision-making (accessed on 11 September 2023).
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278.
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451.
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimer’s Dis. Other Dement. 2018, 33, 500–507.
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270.
- Kerstens, C.; Wildiers, H.P.M.W.; Schroyen, G.; Almela, M.; Mark, R.E.; Lambrecht, M.; Deprez, S.; Sleurs, C. A Systematic Review on the Potential Acceleration of Neurocognitive Aging in Older Cancer Survivors. Cancers 2023, 15, 1215.
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet 2022, 7, e105–e125.
- Chen, L.; Au, E.; Saripella, A.; Kapoor, P.; Yan, E.; Wong, J.; Tang-Wai, D.F.; Gold, D.; Riazi, S.; Suen, C.; et al. Postoperative outcomes in older surgical patients with preoperative cognitive impairment: A systematic review and meta-analysis. J. Clin. Anesth. 2022, 80, 110883.
- Patel, D.; Lunn, A.D.; Smith, A.D.; Lehmann, D.J.; Dorrington, K.L. Cognitive decline in the elderly after surgery and anaesthesia: Results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia 2016, 71, 1144–1152.
- Mahanna-Gabrielli, E.; Schenning, K.J.; Eriksson, L.I.; Browndyke, J.N.; Wright, C.B.; Evered, L.; Scott, D.A.; Wang, N.Y.; Brown, C.H., 4th; Oh, E.; et al. State of the clinical science of perioperative brain health: Report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br. J. Anaesth. 2019, 123, 464–478.
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446.
- Bates, A.; West, M.A.; Jack, S. Framework for prehabilitation services. Br. J. Surg. 2020, 107, e11–e14.
- Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor; National Academies Press: Washington, DC, USA, 2005.
- Varadhan, K.K.; Neal, K.R.; Dejong, C.H.C.; Fearon, K.C.H.; Ljungqvist, O.; Lobo, D.N. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: A meta-analysis of randomized controlled trials. Clin. Nutr. 2010, 29, 434–440.
- Bousquet-Dion, G.; Awasthi, R.; Loiselle, S.È.; Minnella, E.M.; Agnihotram, R.V.; Bergdahl, A.; Carli, F.; Scheede-Bergdahl, C. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: A randomized control trial. Acta Oncol. 2018, 57, 849–859.
- Waterland, J.L.; McCourt, O.; Edbrooke, L.; Granger, C.L.; Ismail, H.; Riedel, B.; Denehy, L. Efficacy of Prehabilitation Including Exercise on Postoperative Outcomes Following Abdominal Cancer Surgery: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 628848.
- Soares, S.M.; Nucci, L.B.; da Silva, M.M.; Campacci, T.C. Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: A randomized controlled trial. Clin. Rehabil. 2013, 27, 616–627.
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.; Burgos, F. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: A randomized blinded controlled trial. Ann. Surg. 2018, 267, 50–56.
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.W.; Jack, S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br. J. Anaesth. 2015, 114, 244–251.
- West, M.A.; Astin, R.; Moyses, H.E.; Cave, J.; White, D.; Levett, D.Z.H.; Bates, A.; Brown, G.; Grocott, M.P.W.; Jack, S. Exercise prehabilitation may lead to augmented tumor regression following neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2019, 58, 588–595.
- Eschke, R.K.; Lampit, A.; Schenk, A.; Javelle, F.; Steindorf, K.; Diel, P.; Bloch, W.; Zimmer, P. Impact of Physical Exercise on Growth and Progression of Cancer in Rodents—A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 35.
- Macmillan, Royal College of Anaesthetists, National Institute for Health and Social Care Research. Principles and Guidance for Prehabilitation within the Management and Support of People with Cancer In Partnership with Acknowledgements. November 2020. Macmillan, London, U.K. Available online: https://www.macmillan.org.uk/dfsmedia/1a6f23537f7f4519bb0cf14c45b2a629/13225-source/prehabilitation-for-people-with-cancer (accessed on 12 November 2023).
- Scheede-Bergdahl, C.; Minnella, E.M.; Carli, F. Multi-modal prehabilitation: Addressing the why, when, what, how, who and where next? Anaesthesia 2019, 74, 20–26.
- Davis, J.F.; van Rooijen, S.J.; Grimmett, C.; West, M.A.; Campbell, A.M.; Awasthi, R.; Slooter, G.D.; Grocott, M.P.; Carli, F.; Jack, S. From Theory to Practice: An International Approach to Establishing Prehabilitation Programmes. Curr. Anesthesiol. Rep. 2022, 12, 129–137.
- Wilson, C.; Colombo, R. Making the Business Case for Implementing Prehabilitation Services. Available online: https://www.accc-cancer.org/docs/documents/management-operations/business-cases/prehab-tool.pdf?sfvrsn=bbfb2fa4_2 (accessed on 11 October 2023).
- Santa Mina, D.; van Rooijen, S.J.; Minnella, E.M.; Alibhai, S.M.H.; Brahmbhatt, P.; Dalton, S.O.; Gillis, C.; Grocott, M.P.W.; Howell, D.; Randall, I.M.; et al. Multiphasic Prehabilitation Across the Cancer Continuum: A Narrative Review and Conceptual Framework. Front. Oncol. 2020, 10, 598425.
- Silver, J.K.; Santa Mina, D.; Bates, A.; Gillis, C.; Silver, E.M.; Hunter, T.L.; Jack, S. Physical and Psychological Health Behavior Changes During the COVID-19 Pandemic that May Inform Surgical Prehabilitation: A Narrative Review. Curr. Anesthesiol. Rep. 2022, 12, 109–124.
- Weston, M.; Weston, K.L.; Prentis, J.M.; Snowden, C.P. High-intensity interval training (HIT) for effective and time-efficient pre-surgical exercise interventions. Perioper. Med. 2016, 5, 2.
- Kidd, T.; Mold, F.; Jones, C.; Ream, E.; Grosvenor, W.; Sund-Levander, M.; Tingström, P.; Carey, N. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019, 19, 184.
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650.
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296.
- Tsimopoulou, I.; Pasquali, S.; Howard, R.; Desai, A.; Gourevitch, D.; Tolosa, I.; Tolosa, I.; Vohra, R. Psychological Prehabilitation Before Cancer Surgery: A Systematic Review. Ann. Surg. Oncol. 2015, 22, 4117–4123.
- Grimmett, C.; Heneka, N.; Chambers, S. Psychological Interventions Prior to Cancer Surgery: A Review of Reviews. Curr. Anesthesiol. Rep. 2022, 12, 78–87.
- Fecher-Jones, I.; Grimmett, C.; Carter, F.J.; Conway, D.H.; Levett, D.Z.H.; Moore, J.A. Surgery school—Who, what, when, and how: Results of a national survey of multidisciplinary teams delivering group preoperative education. Perioper. Med. 2021, 10, 20.
This entry is offline, you can click here to edit this entry!
