Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Infrared (IR) thermography is one of the most promising technologies now available for the early detection of malignant diseases (such as skin and breast cancers). Its significant strengths are the absence of contact and dangerous radiation; it is also a non-invasive and cost-effective technique.
- infrared thermal imaging
- skin cancer
- thermographic technique
1. Introduction
Breast cancer was the most common cancer worldwide in 2022, with more than 2.26 million new diagnoses in women in 2020, whereas melanoma of the skin was the 17th most common cancer, with more than 150,000 new cases [1]. As far as non-melanoma skin cancers are concerned, they are under-reported in the national cancer registry data as they are very common, often under-diagnosed, and commonly treated within primary care. For these reasons, they are often excluded from the reporting of cancer statistics [1]. Additionally, it was estimated that in the EU 27 countries, melanoma accounted for 4% of all new cancer diagnoses (all cancers, excluding non-melanoma skin cancers), with about 16,500 deceased subjects in 2020 [2]. In the same countries, breast cancer was the most diagnosed cancer (13.8%) in 2022 [2], and it claimed 95,800 lives in the same year [2].
Early diagnostic tools could significantly increase survival rates and reduce health-care costs. Apart from invasive examination (biopsy), the visual inspection-based ABCDE criteria are commonly used by expert dermatologists for skin lesions diagnosis as well as dermoscopy. However, at very early stages, melanomas may resemble benign lesions [3,4] and, therefore, visual inspection can sometimes be inaccurate. In regard to breast cancer diagnosis, mammography is considered the gold standard. Notwithstanding, it suffers from low sensitivity for dense breasts in younger women, exposure to X-ray radiation, and relatively high costs [5]. Developing new, accurate, objective, and early diagnostic tools is essential not only to overcome these limitations but also to avoid unnecessary biopsies and consequently reduce the costs of national health-care systems.
Infrared (IR) thermography is one of the most promising technologies now available for the early detection of malignant diseases (such as skin and breast cancers). Its significant strengths are the absence of contact and dangerous radiation; it is also a non-invasive and cost-effective technique. It is based on identifying abnormal thermal patterns on the observed skin surface when compared to the healthy region, as they are recognized as biological risk markers [6]. Surprisingly, the first applications of IR thermography to diagnose breast cancers and melanomas date back to 1956 [7] and 1969 [8], respectively. However, when the observed differences between thermal patterns of cancerous and healthy tissues were subtle, this methodology showed drawbacks and limits due to poor resolution and the scan time of the first IR cameras. Moreover, the absence of standardized measurement procedures and the lack of clinicians adequately trained in the use of IR cameras represented other obstacles to the development of such a methodology. Because of these problems, IR thermography did not become popular as a diagnostic tool [6,9,10,11,12], although in 1982, it was approved as an adjunctive tool for the diagnosis of breast cancer by the Food and Drug Administration in the USA [5]. Later in the 2000s, the performance of IR cameras was significantly improved thanks to the introduction of high-sensitivity uncooled IR detectors (known as focal plane arrays), allowing less pronounced surface temperature variations to be detected. As a consequence of the improvement in IR cameras’ performance along with the increasing computational capability (crucial for both realistic models and artificial intelligence algorithms), IR thermography has gained a renewed interest as a diagnostic tool. Furthermore, the increasing computational capability allows for more accurate numerical models, which are able to predict the complex relationship between surface thermal patterns and the underlying pathophysiological conditions, to be implemented and used both as support for thermographic examination and to provide reliable data useful for training artificial intelligence algorithms. Additionally, relevant information about thermophysical parameters and growth features of tumor lesions can be inferred from surface temperature distributions using inverse techniques.
To the best of the authors’ knowledge, other reviews on this topic are devoted to specific aspects of IR thermography applied for the diagnosis of a particular kind of cancer. Examples are the reviews dedicated to breast cancer detection authored by Kandlikar et al. [13] and by Mashekova et al. [14]. The former addresses both the general aspects of thermography and numerical simulations, highlighting the need for realistic models, while the latter discusses mainly the geometrical aspects of thermal modeling and computer-aided diagnostic tools, underlining the applications of artificial intelligence algorithms. Additionally, the review offered by Ng [6] discusses the camera performance and the environmental requirements, enabling the use of IR thermography as a tool for breast tumor screening. As far as skin cancer detection is concerned, the work by Akther et al. [4] briefly reviews the experimental studies aimed at detecting melanoma, while the review by Verstockt et al. [15] provides an overview of measurement set-ups and technical procedures used for skin cancer diagnosis.
2. Thermographic Techniques for Skin Cancer Diagnosis
Steady-state and dynamic infrared thermography were both suggested for detecting skin cancers. The steady-state thermography requires that the subject reach a sufficient thermal equilibrium with the ambient air contained in the examination room, which should be maintained at a constant temperature during the test. Dynamic thermography usually involves applying thermal stress to the skin region under investigation before acquiring thermal images. Cold stress is often used rather than a hot stimulus. One of the reasons, according to Buzug et al. [23], is that a hot stress can lead to the denaturizing process of proteins when skin temperature exceeds 42 °C.
In 2014, Solivetti et al. [24] applied and compared three different techniques (high-frequency ultrasound, positron emission tomography/computed tomography, and infrared thermography) to examine 15 patients affected by advanced-stage melanoma who presented a total of 52 lesions. The results showed that the ultrasound technique was able to detect all the lesions, while tomography and IR thermography were only able to detect 24 and 15 lesions, respectively. In particular, the thermographic technique results varied according to the lesion size, and they were better for lesions greater than 7 mm. According to these authors, the ultrasound technique integrated with an accurate clinical examination could not be replaced by the other two techniques. However, no thermogram post-processing method was mentioned in their work. The lack of these post-processing techniques, along with the sole visual evaluation of the thermograms, could be the cause of the poor results obtained with IR imaging.
The possibility of distinguishing between melanomas and melanocytic nevi in both steady-state and dynamic conditions was demonstrated by Magalhaes et al. [25] using different machine learning classifiers to separately test the collected thermal images. Thermal acquisitions were performed in a room at 21 °C ± 1 °C and relative humidity less than or equal to 50% using an FLIR E60sc IR camera with a 320 × 240 focal plane array, a noise equivalent temperature difference less than 50 mK at 30 °C, and a measurement uncertainty of ±2%. Also, the quality of the recorded images was ensured by calibrating the camera with a black body source. A period of acclimatization of 10 min was considered satisfactory to reach a good thermal equilibrium to perform the IR imaging in steady-state conditions. In dynamic conditions, a 5 mm diameter aluminum disk with a thickness of 20 mm put in contact for 1 min with the skin lesion was used as a cold stimulus. In the steady-state mode, only one infrared image was acquired, while in the dynamic mode, an image was recorded immediately after the removal of the cold stimulus and the other five at each following minute. Distinctive thermal parameters to characterize the skin lesion were retrieved from the recorded images in both static and dynamic modes.
2.1. Steady-State Thermography
The examination of eleven subjects affected by BCC through steady-state thermography was reported by Flores-Sahagun et al. [26]. The infrared images were recorded using an SAT-S160 infrared camera with a low optical resolution (160 × 120 pixels), a temperature accuracy of ±2%, and a temperature resolution of 0.1 °C. The camera was placed 1 m away from the patient’s skin. However, no information about the acclimatization time was reported in [26]. Dimensionless temperature gradients, defined as [27], were measured between two symmetric regions of the patient’s body in order to compensate the temperature readings with respect to variations in both the metabolism and ambient temperature. The authors concluded that their methodology was capable of detecting BCC in all examined cases, despite the low resolution of the instrumentation used.
A clinical study aimed at establishing the feasibility of distinguishing between benign and malignant lesions using IR thermography was reported by Shada et al. [28] in which 74 patients above the age of 18 were involved. The lesion diameter was used to classify 251 lesions, previously identified using different diagnostic tools, such as clinical diagnosis and tissue biopsy. The sensitivity (percentage of detected true positive) and specificity (percentage of detected true negative) of infrared imaging were computed for each class of diameters. Lesions of a diameter greater than or equal to 1.5 cm yielded the highest sensitivity, while smaller lesions had the lowest sensitivity [28]. Shada et al. also reported high specificity values for all lesion sizes. However, no information about the image acquisition procedure was described in this work.
In 2015, Vardasca et al. [29] showed the feasibility of identifying malignant skin lesions through steady-state thermography by observing the thermal images of 58 patients affected by neoplasms. The subjects were left to reach a sufficient thermal equilibrium with the air contained in an acclimatized room at 21 °C and a relative humidity less than 50% for 15 min before the thermal acquisition. An FLIR A325sc camera having a resolution of 320 × 240 and a precision of 70 mK, placed 80 cm away from the patient, was used to record the thermal images. The authors were able to distinguish benign from malignant skin cancers by computing the thermal contrast between two rectangular regions of interest over the neoplasm region.
To discriminate between benign and malignant skin lesions, Stringasci et al. [30] registered and analyzed steady-state thermal images of clinically similar lesions. The authors compared 100 IR images of BCC with 100 intradermal nevus, 35 images of SCC with 35 actinic keratosis, and 20 melanomas with 20 pigmented seborrheic keratosis. The infrared images were recorded through a FlukeVR FLK-Ti400 IR camera with a resolution of 320 × 240 pixels, a thermal sensitivity of less than 0.05 °C, and precision of ± 2 °C. The IR camera was placed 15 cm away from the lesion, and thermal images were recorder after the patient was hosted for 10 min in an examination room at temperature of 22 °C. Each thermal frame was analyzed in a MATLAB® environment to compute several metrics to be used within a support vector machine classifier to discriminate the lesions. Thermal images showed that actinic keratoses and SCC exhibit clearly distinct average temperatures, while the other pairs of lesions show similar temperatures. However, these similar lesions were successfully discriminated against by the support vector machine classifier using their thermal images. The authors concluded that thermography can become a diagnostic tool for the faster screening of suspicious lesions.
2.2. Dynamic Infrared Thermography
As mentioned previously, dynamic IR thermography involves a cold stimulus to enhance the thermal contrast between healthy and unhealthy skin tissues. However, in the last few years, several authors have suggested other dynamic, well-known techniques in the field of non-destructive testing, such as lock-in thermography and thermal wave imaging. Compared to classical dynamic thermography, these techniques applied for skin cancer diagnosis promise to precisely localize the lesion margins or identify the different development stages of the tumor.
2.2.1. Cold Stimuli for IR Imaging
Different skin cooling methods were investigated and compared in the scientific literature. The results obtained provide useful information when selecting a suitable cold stimulus in terms of the effectiveness of the cooling and the characteristics of the lesion.
Zenzie et al. [31] compared the skin cooling achieved through the spray and contact methods, both theoretically and experimentally, using an in vitro model. They concluded that both methods provide efficient skin cooling and that target depth, cost, and safety should be the deciding factors when choosing the cooling method.
Deng and Liu [32] showed that the thermal contrast between skin lesions and healthy skin can be enhanced by inducing the evaporation of water and 75% medical ethanol sprayed on the skin surface. The authors performed both numerical calculations and infrared imaging experiments on a human forearm. They found that the vascular pattern of the forearm became clearly visible several minutes after the induced evaporation. Therefore, according to the author’s point of view, induced evaporation is an effective method for enhancing the skin’s thermal contrast.
For three different skin cooling methods, Cheng and Herman [33] performed a computational analysis to properly select the cooling temperature, time, and cooling depth in order to maximize the thermal contrast between a lesion and healthy skin when using dynamic infrared imaging. These authors investigated idealized contact cooling at constant temperature, a water-soaked cotton patch, and convective cooling through a cold air flow or immersion into a liquid. They found that, for the three methods, the cooling effect takes less than 30 s to reach a depth of 4 mm. Also, when a cooling temperature of about 20 °C is applied, effective skin cooling is achieved; in particular, the cooling effect can reach the lesion depth within 2 min without causing discomfort to the patient. Moreover, Cheng and Herman considered cooling durations varying from 5 to 120 s; as a consequence, they studied two thermal recovery behaviors: one for short cooling times (less than 30 s) and another for longer cooling times. In particular, shorter cooling times lead to a cooling penetration depth less than 4 mm, with a maximum thermal contrast appearing within the first few seconds of the thermal recovery phase; while longer cooling durations give a lower maximum appearing 20–45 s after the removal of cold stimulus. The authors concluded that the cooling duration could be further adjusted by considering the characteristics of the lesion.
To induce deeper cooling penetration and, therefore, better thermal contrast when using dynamic IR thermography, a constant-temperature active cooling device was numerically investigated by Gomboc et al. [34]. This set-up involves a metal disk put in direct contact with the lesion and the surrounding tissue; also, a thermoelectric device (Peltier cell) mounted over the disk top provides the cooling effect. The aim was to ensure that the disk temperature was constant as possible during the cooling phase. The authors concluded that to realize faster regulation and more constant cooling temperature, the use of a thin metal disc is preferable to a thick disk. According to the authors, the set-up may be used in any tumor stage as the lesion dimensions do not affect the cooling temperature.
Recently, Verstockt et al. [35] compared different cooling techniques useful in skin cancer diagnosis when using dynamic IR thermography: conductive cooling (aluminum medal, gel pack, and ice), evaporating cooling (alcohol spray), and convective cooling (Vortex tube and Zimmer Crio 6). Skin-mimicking agar phantoms with similar thermal properties to human skin and resembling both flat skin and an ulcerating skin lesion were used for the experimental tests. The skin phantoms were cooled for a period of 60 s, and the successive reheating process was recorded by an IR camera and two embedded resistance temperature detectors to assess the cooling penetration over time. To compare the cooling techniques, the authors defined a decision matrix considering different criteria, such as uniform cooling, repeatability, obstructing the view, cooling efficiency, workload per patient, patient comfort, use in a clinical setting, noise exposure, consumables, additional equipment, and price. The most suitable cooling method for skin cancer diagnosis was the technique that reached the highest weighted score. The authors concluded that convective cooling techniques create a more uniform cooling effect than conductive techniques, which are effective only for cooling flat objects; indeed, when they are applied to bulging skin, uniform cooling is more challenging to obtain. According to the authors, cooling methods involving ice or alcohol are unsuitable when using infrared thermography because they can compromise the accuracy of the temperature readings as they affect both the infrared radiation and the thermal camera’s view. Based on their decision matrix, Verstockt et al. stated that the Zimmer Cryo 6 cooler is the best cooling method thanks to its ability to uniformly cool the skin, the consistent conditioning of cold air, high cooling efficiency at the airflow temperature of −30 °C, and unnecessary consumables; on the other hand, the disadvantage of this cooling device is its cost.
Cryogenic cooling of the skin was also applied in the thermographic study, aided by numerical simulations and recently published by Verstockt et al. [36]. The authors showed that the choice of an appropriate cooling method, a cooling sequence, and an optimized experimental set-up leads to an improvement in thermal contrast.
2.2.2. Dynamic IR Thermography Using a Cold Provocation
Dynamic IR thermography was successfully applied by Buzug et al. [23] to detect a BCC. In particular, a cool gel pack was used to cool down to 27 °C on a skin area of 10 × 10 cm2. The thermal recovery was then recorded by an FLIR SC 3000 camera with a temperature resolution of 0.03 K for 5 min with a frame rate of 1 Hz. The involuntary movements of the patients were compensated by detecting the bore holes of a fiducial marker in the frame sequence using the generalized Hough transformation. In 2006, the work of Buzug et al. revealed dynamic thermography as a powerful diagnostic tool.
In 2009, Santa Cruz et al. began an experimental program aimed at investigating the feasibility of dynamic infrared thermography for follow-up melanoma patients treated with Boron Neutron Capture Therapy (BNCT) [37]. The patients remained at rest for 15–20 min in order to allow the examined region to reach an approximate thermal equilibrium with the environment. Then, the initial temperature distribution was recorded for 30 s (basal study); after that, an immersion in water at 15 °C for 2 min or an alcohol spray followed by fan currents over the region (to induce cooling by forced evaporation) were used as cold stimuli. Again, a second thermal acquisition was recorded for 3 min or more. In order to compare the steady and post-stimulus temperature distributions, the examined region was immobilized, and anatomical landmarks were used for image acquisition. In the early phase of this study, two patients were followed up for 30 weeks after the BNCT treatment, revealing dynamic thermography as a useful tool for melanoma monitoring during BNCT treatments as well as for optimizing the treatment itself.
Cetingul and Herman developed a thermal infrared imaging system aimed at early diagnosing malignant pigmented skin lesions [38]. This system allows for small temperature differences on the skin surface to be accurately measured. In their pilot study, 37 patients with a pigmented lesion with a clinical indication for biopsy were tested. A 50 mm diameter skin region containing the lesion was cooled by a stream of cold air at 15 °C for 1 min. The thermal recovery phase was recorded for 3–4 min by an infrared camera (equipped with a 320 × 256 pixel InSb focal plane array and having a sensitivity of 0.025 °C) located 30 cm away from the patient. The acquisition frequency was set at 2 s. To analyze the infrared images, an involuntary movement correction and an interactive lesion segmentation were applied. In particular, to align the recorded thermal image sequence, the corners of an adhesive marker were used as landmarks in a quadratic motion model. Then, an interactive image segmentation algorithm was used to create a mask image delineating the lesion, which in turn was used to identify the lesion region in each of the thermal images. Cetingul and Herman reported the detection of two melanoma cases at a very early stage, for which temperature differences of 0.5 °C and 2.2 °C, respectively, between the lesion and healthy tissue were observed.
Thirty-six chronic sun-exposed subjects affected by 87 actinic keratosis and 48 BCC lesions were examined by Di Carlo et al. [39] using both dynamic IR thermography and dermoscopy. A cold stress at 5 °C on the skin area containing the lesion was applied for 20 s, and after that, the thermal recovery of this region was recorded by an FLIR3000 Thermocam. The thermal images showed well-distinct thermal patterns for the two kinds of lesions: all the actinic keratosis lesions exhibited a hyperthermic pattern, while the BCC lesions showed a hypothermic one. On the contrary, dermoscopic examination a 22% of cases for which no diagnostic indications were achievable. However, the authors stated that to confirm IR thermography as a diagnostic tool, a larger sample of subjects has to be enrolled.
Baek et al. [40] distinguished pigmented BCC and seborrheic keratosis by comparing the thermal recovery patterns of skin lesions and healthy skin recorded after the application of both hot and cold stress supplied with a thermoelectric device. Thermal images were recorded from a group of 37 patients affected by pigmented BCC (22 individuals) and seborrheic keratosis (15 individuals) and enrolled to undergo an original IR imaging procedure. After 5–10 min of acclimatization in a controlled temperature room at 23 °C and 50 ± 5% relative humidity, the skin lesion was initially heated up to 40 °C; after that, the hot stimulus was immediately removed and the thermal response of the lesion was recorded for five minutes through an FLIRVR A615 IR camera (with an optical resolution of 640 × 480 pixels and a noise equivalent temperature difference less than 0.05 °C at 30 °C). After 5 min of rest, the same lesion was cooled to 15 °C, and thermal images were acquired for the other five minutes after removing the cold stimulus. The same procedure was also applied to the healthy skin. The classification method was based on the fact that pigmented BCC showed faster thermal recovery than normal skin, whereas no significant differences between seborrheic keratosis lesions and healthy skin appeared when comparing their thermal responses.
In 2015, Godoy et al. suggested an analysis method for skin cancer screening using dynamic infrared thermography [41]. About one hundred subjects underwent the suggested method. A cold air flow produced by a Ranque–Hilsch vortex tube was used to cool the subject’s skin around the suspected lesion for 30 s. Moreover, the examination room was controlled to be between 20 and 22 °C. Thermal images were acquired for a total of 2 min during both the cooling and the thermal recovery phases using a long-wave infrared camera with a 320 × 256 focal-plane array. A squared infrared marker was used to correct the involuntary movement of the subject using the Harris corner detector algorithm, and an affine transformation matrix was used to map the movements between consecutive frames. The effect of the non-uniform cooling was also considered by judiciously selecting pixels with the same initial temperature. The authors reported that their method allows 95% of the malignant cases to be correctly classified, while more than 83% of the benign lesions would be identified without the need for a biopsy.
A group of 30 patients affected by actinic keratosis were periodically monitored after the surgical removal of the lesions by Laino et al. [42], using dynamic IR thermography to evaluate the effectiveness of treatment with Eryfotona® in reducing the hyperthermic halo typical of this lesion. The authors employed an FLIR3000 IR camera together with patented equipment to provide the thermal stimulus. In particular, it consisted of an insulated tank containing a mixture of alcohol and water and connected, with a two-way tube, to a rubber balloon, which was put in contact with the skin. The temperature of the mixture can be set from 0 °C to 40 °C by means of a heating–cooling system embedded within the tank walls. In their study, the patient’s skin was cooled for 20 s until it reached a temperature of 5 °C. The area of the hyperthermic region and the thermal recovery time, i.e., the time the skin takes to come back to steady-state conditions, were the parameters monitored by the authors.
Dynamic IR thermography was also applied to monitor six patients affected by BCC who underwent photodynamic therapy (PDT) [43]. However, in this study carried out by Cholewka et al., a cold stimulus was not applied; on the contrary, during the photodynamic treatment, the patient’s skin was illuminated by a diode laser source. Thermal images were recorded using an FLIR Thermovision Camera E60 (sensitivity of 50 mK), placed 0.5 m away from the skin lesion, before and immediately after illumination, as well as after 15, 30, and 45 min. Tests were performed in a special room whose temperature was maintained at 23 ± 1 °C. According to the authors, knowledge of the observable changes in the temperature gradient of the lesion due to the photodynamic treatment can provide useful information about chemical and physiological processes occurring during therapy.
2.2.3. Lock-in Thermography
Lock-in thermal imaging (LIT), first developed in the 1990s for non-destructive testing of materials, was first used for dermatological applications by Bonmarine and Le Gal [44]. The experimental set-up suggested by the authors foresees the use of a temperature-modulated airflow to periodically stimulate the skin surface. This set-up consists of a cold air device in series with a resistive wire (whose voltage is periodically modulated) to create a temperature-modulated air flow. Moreover, thermal images were recorded by an infrared camera synchronized with the air flow modulation.
Then, the acquired thermal frames had to be demodulated according to the digital lock-in principle. Applying this procedure yields a phase and an amplitude image; the former is a map of the phase angles between the thermal stimulus and the temperature response of the skin, while the second image is related to the dissipated power at the skin surface. The main advantage of LIT compared to steady-state and dynamic IR thermography is its ability to detect very small temperature gradients even when a noisy background occurs. In fact, this method is able to reject irrelevant thermal signals coming from the metabolic and circulatory variations occurring in the subcutaneous tissues [44]. Also, the suppression of the lateral heat spreading achievable in the phase image allows the lesion and its margins to be localized accurately. Note that the same accuracy is not achievable using steady-state thermography. However, the results of the application of this method were reported by the authors only for benign skin lesions.
2.2.4. Thermal Wave Imaging
The feasibility of the frequency-modulated thermal wave imaging (FMTWI) technique used for the detection and distinction of skin cancer stages was theoretically proved for the first time by Bhowmik et al. [45] in 2005. The FMTWI technique foresees a controlled heating of the skin surface through a frequency-modulated heat flux of the desired magnitude and bandwidth, as shown in Figure 1. As a consequence, a highly diffused thermal wave propagates within the tissue, leading to time-varying temperature distributions. During the active heating, the temperature of the irradiated surface was recorded by an IR camera [45]. This method can be applied to thermally probe skin lesions at different depths using a single experimental test. Additionally, it requires a low peak power of the incident heat flux, and it is faster than other imaging techniques requiring periodically amplitude-modulated thermal excitation, such as LIT. The results showed that the FMTWI technique is suitable for the detection and differentiation of melanomas with large volumes rather than melanomas at the earliest development stages (with small volumes). Finally, by extracting the phase information from the simulated thermograms, the authors reported that the phase images have the advantage of allowing different development stages of melanoma to be clearly detected, compared to raw thermograms recorded by simply applying dynamic IR thermography.
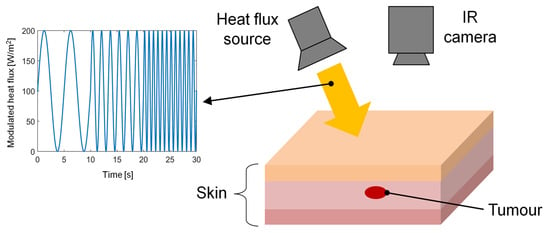
Figure 1. Schematic of the experimental apparatus of thermal wave imaging technique.
2.2.5. Literature Survey on Skin Cancer Diagnosis
The key findings and measurement protocols used in the scientific research mentioned in the above sections are summarized in Table 1.
Table 1. Summary of scientific research for skin cancer diagnosis using IR thermography.
| Study | Thermal Imaging Technique |
Sample Size and Lesion Type | Camera Specifications | Cooling/Heating Methods/ Acclimatization Information |
Findings/Results |
|---|---|---|---|---|---|
| Gonzalez et al. [16] | Infrared Thermography. |
30 patients (6 melanomas, 18 BCC, and 6 SCC). | FLIR T400 IR camera (320 × 240 FPA, spectral range 7.5–13 μm, thermal sensitivity 50 mK at 30 °C). | … | Differentiated BCC, SCC, and melanoma based on temperature differences. Correlation with vascularity. |
| Flores -Sahagun et al. [26] | Steady-state Thermography. | 11 subjects (BCC). | SAT-S160 Infrared camera (160 × 120 pixels, temp. accuracy ± 2%, temp. resolution 0.1 °C). | … | Detected BCC despite low camera resolution. |
| Shada et al. [28] | Steady-state Thermography. | 74 patients (various lesions). | Camera specifications not provided. | … | Evaluated sensitivity and specificity based on lesion diameter. |
| Vardasca et al. [29] | Steady-state Thermography. | 58 patients (neoplasms). | FLIR A325sc camera (320 × 240 resolution, precision 70 mK). | 15 min acclimatization. | Distinguished benign from malignant skin cancers based on thermal contrast. |
| Stringasci et al. [30] | Steady-state Thermography. | 100 cases each (various lesions). | FlukeVR FLK-Ti400 IR camera (320 × 240 pixels, thermal sensitivity < 0.05 °C, precision ± 2 °C). | 10 min acclimatization. | Used SVM classifier to discriminate lesions; identified clear temperature differences for some lesions. |
| Buzug et al. [23] | Dynamic IR Thermography (cold). | … | FLIR SC 3000 camera (temp. resolution 0.03 K). | Cool gel pack. | Detected BCC using dynamic thermography with cold stimulus. |
| Santa Cruz et al. [37] | Dynamic IR Thermography (cold). | … | Camera specifications not provided. | Water immersion or alcohol spray with fan currents. | Used dynamic thermography to monitor melanoma patients during BNCT. |
| Cetingul and Herman [38] | Dynamic IR Thermography (Cold) | 37 patients (pigmented lesions). | Unspecified IR Camera (320 × 256 pixel InSb FPA and sensitivity of 0.025 °C). | Stream of cold air at 15 °C. | Detected early-stage melanoma cases with a stream of cold air as a cooling stimulus. |
| Di Carlo et al. [39] | Dynamic IR Thermography (cold). | 36 patients (87 actinic keratosis and 48 BCC). | FLIR3000 Thermocam. | Cold stress at 5 °C for 20 s. | Distinguished thermal patterns of actinic keratosis and BCC. |
| Baek et al. [40] | Dynamic IR Thermography (hot and cold). | 37 patients (22 BCC and 15 seborrheic keratosis). | FLIRVR A615 IR camera (640 × 480 pixels, noise equivalent temp. difference < 0.05 °C at 30 °C). | 5–10 min acclimatization, hot stress up to 40 °C, and cold stress to 15 °C after 5 min rest. | Distinguished pigmented BCC and seborrheic keratosis. |
| Godoy et al. [41] | Dynamic IR Thermography (cold). | About 100 subjects. | Long-wave infrared camera (320 × 256 FPA). | Cold air flow produced by Ranque–Hilsch vortex tube. | Successfully classified malignant cases using a cold air flow cooling stimulus. |
| Laino et al. [42] | Dynamic IR Thermography (cold). | 30 patients (actinic keratosis). | FLIR3000 IR camera. | Alcohol and water mixture cooling. | Monitored effectiveness of actinic keratosis treatment with dynamic IR thermography. |
| Cholewka et al. [43] | Dynamic IR Thermography (laser). | 6 patients (BCC). | FLIR Thermovision Camera E60 (sensitivity 50 mK). | Laser illumination (active thermography). | Investigated temperature gradient changes due to photodynamic therapy for BCC. |
| Bonma- rine and Le Gal [44] |
Lock-in Thermography. | … | IR camera and temperature-modulated airflow. | Temperature-modulated air flow synchronized with the camera. | Used lock-in thermography for dermatological applications, reported results for benign skin lesions. |
| Bhowmik et al. [45] | Thermal Wave Imaging (FMTWI). | … | IR camera and controlled heating. | Controlled heating of the skin surface. | Theoretical feasibility of FMTWI for detection and differentiation of melanoma stages. |
This entry is adapted from the peer-reviewed paper 10.3390/app14041603
This entry is offline, you can click here to edit this entry!
