Groundwater constitutes a vital source of freshwater, accounting for roughly 95% of the total available freshwater resources on Earth. It is utilized not only for daily water needs but also for agricultural irrigation, industrial purposes, ecological recharge, and power generation.
- high-arsenic groundwater
- worldwide scale
- in situ remediation of arsenic
- human health risk assessment
1. Introduction
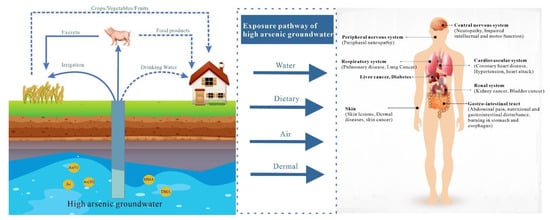
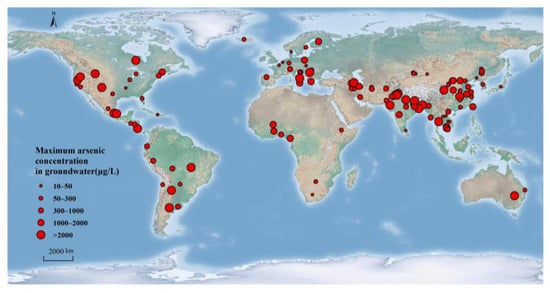
2. Global Distribution of Geogenic High-Arsenic Groundwater
| Country | Study Area | Max As conc. (µg/L) | Samples | Environmental Condition and/or Enrichment Mechanism | References |
|---|---|---|---|---|---|
| Afghanistan | Ghazni and maidan Wardak provinces | 990 | 746 | The weathering and leaching action | [39] |
| Argentina | Santiago del Estero Province | 14,969 | 40 | Volcanic ash sedimentary environment; agricultural irrigation | [40] |
| La Pampa | 5300 | 44 | The geological factors; weathering of volcanic ash and loess; oxidizing condition | [41] | |
| Australia | Stuarts Point coastal | 85 | 140 | Desorption of As from Al-hydroxides and As-enriched Fe-oxyhydroxides; high concentrations of HCO3− and PO4− | [42] |
| Bangladesh | Noakhali | 4730 | 52,202 | Eroded by flood plain rivers | [25] |
| Bolivia | 364 | 24 | The alteration of volcanic rocks; evaporation and redox reactions | [43] | |
| Botswana | Botswana | 116 | 20 | Delta; evaporation concentration; weakly alkaline environment; pH 6.29–8.60 | [44] |
| Brazil | 2980 | Anthropogenic; volcanic activity and weathering of rocks | [43] | ||
| Burkina Faso | 1630 | 45 | Zones of gold mineralization in volcano-sedimentary rocks | [45] | |
| China | Datong Basin | 1932 | 1022 | The weak alkaline reductive environment; high HCO3− concentration; water–rock interactions | [46] |
| Hetao Basin | 572 | 63 | The reducing conditions; the dissolved organic; the competitive effects of other anions | [47] | |
| Jianghan Basin | 2330 | 34 | The high HCO3− concentrations; microorganisms and exogenous substances; the seasonal variation; strongly reducing environment; reducing environment | [48] | |
| Taiwan (Lanyang and Chianan Plain) | 1010 | Alluvial plain; high DOC; strong reducing conditions | [49] | ||
| Tarim Basin | 91.2 | 233 | Reducing environment; the dissolved organic; reductive dissolution release; | [50] | |
| Yinchuan | 177 | 92 | Agricultural irrigation; the reductive dissolution of Fe oxides; the high PO4− concentrations | [51] | |
| Pearl River Delta | 161 | 18 | Reductive environment; the high NH4+ concentrations; high concentrations of NH4+ and organic matter | [52] | |
| Cambodia | 1610 | 207 | Holocene alluvial sediments; reducing environment | [53] | |
| Costa Rica | Northern Costa Rica | 29,100 | 35 | Associated with the volcanic rock | [43] |
| Czech Republic | Mokrsko | 1690 | 62 | pH> 9 | [54] |
| Ecuador | 969 | 67 | In hot springs | [43] | |
| Ethiopia | Southwestern Ethiopia | 184.5 | 44 | pH < 7 | [55] |
| Ghana |
|
1760 | 357 | Spillages of the mines; pH 4.8–6.99 | [56] |
| Hungary | Southern Hungary | 260 | 73 | At a depth of 0.8–2.4 km and containing CH4 | [57] |
| India | Bhair | 1466 | 1365 | Ganga Plain; Holocene newer alluvium and the Pleistocene older alluvium | [58] |
| Shahpur block, Bhojpur district, Bihar state | 1805 | 4704 | Ganges plain | [28] | |
| Punjab | 3192 | 4780 | Alluvial aquifers | [58] | |
| Iran | Kurdistan Some villages | 1500 | 27 | Mining and sedimentary environment | [59] |
| East Azarbaijan-Tabriz Plain | 2000 | 18 | Hydrogeological and environmental reducing conditions | ||
| Ardabil-A city | 5834 | 163 | Interaction of hydrothermal fluids with the rocks and geogenic source-geological structure | ||
| Mazandar an-Haraz River | 110 | 20 | Geogenic source and mining | ||
| Tabas South Khorasan | 53 | 29 | Weathering | ||
| Razavi Khorasan Chelpu Kashmar | 606 | 12 | GeogenicOrigin sedimentary environment | ||
| Isfahan Mutehgold mining district | 1061 | 17 | Weathering and mining | ||
| Japan | 38 | 136 | Reducing environment and factory blowdown | [26] | |
| Korea | Geumsan County | 113 | 150 | Oxidation reaction of sulfide minerals in metasedimentary rocks and desorption process under high pH conditions | [60] |
| Nigeria | Warri-Port Harcourt, Ogun State, Kaduna | 750 | 20 | Alluvial sediments, , slightly acidic | [16] |
| Pakistan | Kasur, Shhiwal, Bahawalpur, and Rahim Yar Khan | 3090 | 395 | Irrigation and factory sewage | [61] |
| Lahore municipality | 85 | 41 | Topsoil and extensive irrigation of unconfined aquifers, reductive dissolution | [32] | |
| Mailsi | 812 | 44 | Human activity | [49] | |
| Paraguay |
|
120 | 37 | Human activity and volcanic ash deposition environment | [43] |
| Lao PDR | Vientiane | 24.4 | 3 | Reducing environment | [17] |
| Borikhamxay | 30 | 7 | Reducing environment | ||
| Champasack | 25.6 | 27 | Reducing environment | ||
| Attapeu | 31.6 | 10 | Reducing environment | ||
| Myanmar | Ayeyarwady | 630 | 55 | Reductive dissolution of Fe oxyhydroxides | [49] |
| Mexico | La Laguna Region | 5000 | 29 | Adsorption or coprecipitation on iron oxides, clay-mineral surfaces, and organic carbon | |
| Zacatecas | 75.4 | 182 | Geological origin, water–rock interaction | [49] | |
| Nepal | Nawalparasi | 2620 | 18,000 | Seasons and climate change, water–rock interaction | |
| Pakistan | Larkana Sindh, | 318 | 58 | pH 6.8–8.1 | [62] |
| Punjab | 655 | 141 | pH 7.0–9.3 | [63] | |
| Spain | Duero Cenozoic Basin | 613 | 514 | pH 5.87–1.58 | [64] |
| Thailand | Suphan Buri | 5000 | 21 | pH 5.20–5.90; Eh 250–370 mV | [16] |
| USA | San Joaquin Valley, California | 148.5 | 4983 | Arid and semi-arid basins; alluvial, fluvial, and lacustrine deposits; pH > 7.8; reducing conditions | [65] |
| Lahontan Valley, in Churchill County, Nevada | 4100 | 59 | Lacustrine sediments | [66] | |
| Vietnam | Mekong Delta | 850 | 109 | pH 7.22–8.63 | [49] |
In Africa, high-As groundwater has been found in only a few areas across the continent, primarily in the western and southern regions, more due to insufficient research rather than a shortage of problems [67]. Twenty countries in Africa have recorded high concentrations of arsenic in groundwater, including Botswana, Burkina Faso, Ethiopia, and Ghana [67]. The maximum concentration of As in groundwater in Burkina Faso was 1630 μg/L, while an analogous maximum concentration of 1760 μg/L was detected in groundwater in Ghana [56,73].
This entry is adapted from the peer-reviewed paper 10.3390/w16030478
References
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O.; Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259-284, .
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O.; Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259-284, .
- Burri, N.M.; Weatherl, R.; Moeck, C.; Schirmer, M. A Review of Threats to Groundwater Quality in the Anthropocene. Sci. Total Environ. 2019, 684, 136–154.
- ATSDR. The ATSDR 2019 Substance Priority List, Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 5 November 2023).
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and Temporal Variations of Groundwater Arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127.
- Gorchev, H.G.; Ozolins, G. WHO Guidelines for Drinking-Water Quality. WHO Chron. 1984, 38, 104–108.
- Thakur, J.K.; Thakur, R.K.; Ramanathan, A.L.; Kumar, M.; Singh, S.K. Arsenic Contamination of Groundwater in Nepal—An Overview. Water 2011, 3, 1–20.
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A Review on Decontamination of Arsenic-Contained Water by Electrocoagulation: Reactor Configurations and Operating Cost along with Removal Mechanisms. Environ. Technol. Innov. 2020, 17, 100519.
- Rahaman, M.S.; Rahaman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental Arsenic Exposure and Its Contribution to Human Diseases, Toxicity Mechanism and Management. Environ. Pollut. 2021, 289, 117940.
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and Management of Arsenic Pollution in Groundwater: A Comprehensive Appraisal of Recent Global Scenario, Human Health Impacts, Sustainable Field-Scale Treatment Technologies. J. Environ. Chem. Eng. 2021, 9, 105203.
- Hung, D.Q.; Nekrassova, O.; Compton, R.G. Analytical Methods for Inorganic Arsenic in Water: A Review. Talanta 2004, 64, 269–277.
- Kumaresan, M.; Riyazuddin, P. Overview of Speciation Chemistry of Arsenic. Curr. Sci. 2001, 80, 837–846.
- Kalman, J.; Smith, B.D.; Bury, N.R.; Rainbow, P.S. Biodynamic Modelling of the Bioaccumulation of Trace Metals (Ag, As and Zn) by an Infaunal Estuarine Invertebrate, the Clam Scrobicularia Plana. Aquat. Toxicol. 2014, 154, 121–130.
- Ansari, M.A.; Saravana Kumar, U.; Noble, J.; Akhtar, N.; Akhtar, M.A.; Deodhar, A. Isotope Hydrology Tools in the Assessment of Arsenic Contamination in Groundwater: An Overview. Chemosphere 2023, 340, 139898.
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235.
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079.
- Cho, K.H.; Sthiannopkao, S.; Pachepsky, Y.A.; Kim, K.-W.; Kim, J.H. Prediction of Contamination Potential of Groundwater Arsenic in Cambodia, Laos, and Thailand Using Artificial Neural Network. Water Res. 2011, 45, 5535–5544.
- Buschmann, J.; Berg, M.; Stengel, C.; Sampson, M.L. Arsenic and Manganese Contamination of Drinking Water Resources in Cambodia: Coincidence of Risk Areas with Low Relief Topography. Environ. Sci. Technol. 2007, 41, 2146–2152.
- Van Geen, A.; Ahmed, E.B.; Pitcher, L.; Mey, J.L.; Ahsan, H.; Graziano, J.H.; Ahmed, K.M. Comparison of Two Blanket Surveys of Arsenic in Tubewells Conducted 12years Apart in a 25km2 Area of Bangladesh. Sci. Total Environ. 2014, 488–489, 484–492.
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Trang, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and Temporal Evolution of Groundwater Arsenic Contamination in the Red River Delta, Vietnam: Interplay of Mobilisation and Retardation Processes. Sci. Total Environ. 2020, 717, 137143.
- Winkel, L.; Berg, M.; Amini, M.; Hug, S.J.; Annette Johnson, C. Predicting Groundwater Arsenic Contamination in Southeast Asia from Surface Parameters. Nat. Geosci. 2008, 1, 536–542.
- Brammer, H.; Ravenscroft, P. Arsenic in Groundwater: A Threat to Sustainable Agriculture in South and South-East Asia. Environ. Int. 2009, 35, 647–654.
- Ganguli, S.; Rifat, M.A.H.; Das, D.; Islam, S.; Islam, M.N. Groundwater Pollution in Bangladesh: A Review. Grassroots J. Nat. Resour. 2021, 04, 115–145.
- Bangladesh Bureau of Statistics. Bangladesh National Drinking Water Quality Survey of 2009; Bangladesh Bureau of Statistics: Dhaka, Bangladesh, 2011.
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Chandra Mukherjee, S.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S.; et al. Status of Groundwater Arsenic Contamination in Bangladesh: A 14-Year Study Report. Water Res. 2010, 44, 5789–5802.
- Tashdedul, H.M.; Reyes, N.J.D.G.; Jeon, M.; Kim, L.-H. Current Status and Technologies for Treating Groundwater Arsenic Pollution in Bangladesh. J. Wetl. Res. 2022, 24, 142–154.
- Chakraborti, D.; Rahman, M.M.; Chatterjee, A.; Das, D.; Das, B.; Nayak, B.; Pal, A.; Chowdhury, U.K.; Ahmed, S.; Biswas, B.K.; et al. Fate of over 480 Million Inhabitants Living in Arsenic and Fluoride Endemic Indian Districts: Magnitude, Health, Socio-Economic Effects and Mitigation Approaches. J. Trace Elem. Med. Biol. 2016, 38, 33–45.
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic Contamination of Groundwater and Its Induced Health Effects in Shahpur Block, Bhojpur District, Bihar State, India: Risk Evaluation. Environ. Sci. Pollut. R. 2016, 23, 9492–9504.
- Bindal, S.; Singh, C.K. Predicting Groundwater Arsenic Contamination: Regions at Risk in Highest Populated State of India. Water Res. 2019, 159, 65–76.
- Chakraborti, D.; Ghorai, S.; Das, B.; Pal, A.; Nayak, B.; Shah, B. Arsenic Exposure through Groundwater to the Rural and Urban Population in the Allahabad-Kanpur Track in the Upper Ganga Plain. J. Environ. Monit. JEM 2009, 11, 1455–1459.
- Mukherjee, A.; Verma, S.; Gupta, S.; Henke, K.R.; Bhattacharya, P. Influence of Tectonics, Sedimentation and Aqueous Flow Cycles on the Origin of Global Groundwater Arsenic: Paradigms from Three Continents. J. Hydrol. 2014, 518, 284–299.
- Podgorski, J.E.; Eqani, S.A.M.A.S.; Khanam, T.; Ullah, R.; Shen, H.; Berg, M. Extensive Arsenic Contamination in High-pH Unconfined Aquifers in the Indus Valley. Sci. Adv. 2017, 3, e1700935.
- Shahid, M. A Meta-Analysis of the Distribution, Sources and Health Risks of Arsenic-Contaminated Groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319.
- Cao, W.G.; Zhang, Z.; Guo, H.M.; Fu, Y.; Gao, Z.P.; Nan, T.; Ren, Y.; Li, Z.Y. Spatial Distribution and Controlling Mechanisms of High Fluoride Groundwater in the Coastal Plain of Bohai Rim, North China. J. Hydrol. 2023, 617, 128952.
- Wen, D.G.; Zhang, F.C.; Zhang, E.Y.; Wang, C.; Han, S.B.; Zheng, Y. Arsenic, Fluoride and Iodine in Groundwater of China. J. Geochem. Explor. 2013, 135, 1–21.
- He, X.D.; Li, P.Y.; Ji, Y.J.; Wang, Y.H.; Su, Z.M.; Elumalai, V. Groundwater Arsenic and Fluoride and Associated Arsenicosis and Fluorosis in China: Occurrence, Distribution and Management. Expo. Health 2020, 12, 355–368.
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A Review of High Arsenic Groundwater in Mainland and Taiwan, China: Distribution, Characteristics and Geochemical Processes. Appl. Geochem. 2014, 41, 196–217.
- Rodríguez-Lado, L.; Sun, G.F.; Berg, M.; Zhang, Q.; Xue, H.B.; Zheng, Q.M.; Johnson, C.A. Groundwater Arsenic Contamination Throughout China. Science 2013, 341, 866–868.
- Saffi, M.H.; Eqrar, M. Arsenic Contamination of Groundwater in Ghazni and Maidan Wardak Provinces: Afghanistan. In Arsenic Research and Global Sustainability: Proceedings of the Sixth International Congress on Arsenic in the Environment (As2016), Stockholm, Sweden, 19–23 June 2016; CRC Press: Boca Raton, FL, USA, 2016; pp. 41–42. ISBN 978-1-138-02941-5.
- Bhattacharya, P.; Claesson, M.; Bundschuh, J.; Sracek, O.; Fagerberg, J.; Jacks, G.; Martin, R.A.; Storniolo, A. del R.; Thir, J.M. Distribution and Mobility of Arsenic in the Río Dulce Alluvial Aquifers in Santiago Del Estero Province, Argentina. Sci. Total Environ. 2006, 358, 97–120.
- Aullón Alcaine, A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.-P.; Mörth, C.-M.; Sracek, O.; Ahmad, A.; Bhattacharya, P. Hydrogeochemical Controls on the Mobility of Arsenic, Fluoride and Other Geogenic Co-Contaminants in the Shallow Aquifers of Northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671.
- Smith, J.V.S.; Jankowski, J.; Sammut, J. Vertical Distribution of As(III) and As(V) in a Coastal Sandy Aquifer: Factors Controlling the Concentration and Speciation of Arsenic in the Stuarts Point Groundwater System, Northern New South Wales, Australia. Appl. Geochem. 2003, 18, 1479–1496.
- Bundschuh, J.; Armienta, M.A.; Morales-Simfors, N.; Alam, M.A.; López, D.L.; Delgado Quezada, V.; Dietrich, S.; Schneider, J.; Tapia, J.; Sracek, O.; et al. Arsenic in Latin America: New Findings on Source, Mobilization and Mobility in Human Environments in 20 Countries Based on Decadal Research 2010-2020. Crit. Rev. Env. Sci. Tec. 2021, 51, 1727–1865.
- Huntsman-Mapila, P.; Mapila, T.; Letshwenyo, M.; Wolski, P.; Hemond, C. Characterization of Arsenic Occurrence in the Water and Sediments of the Okavango Delta, NW Botswana. Appl. Geochem. 2006, 21, 1376–1391.
- Smedley, P.L.; Knudsen, J.; Maiga, D. Arsenic in Groundwater from Mineralised Proterozoic Basement Rocks of Burkina Faso. Appl. Geochem. 2007, 22, 1074–1092.
- He, X.D.; Li, P.Y.; Wu, J.H.; Wei, M.J.; Ren, X.F.; Wang, D. Poor Groundwater Quality and High Potential Health Risks in the Datong Basin, Northern China: Research from Published Data. Environ. Geochem. Health 2021, 43, 791–812.
- Guo, H.; Yang, S.; Tang, X.; Li, Y.; Shen, Z. Groundwater Geochemistry and Its Implications for Arsenic Mobilization in Shallow Aquifers of the Hetao Basin, Inner Mongolia. Sci. Total Environ. 2008, 393, 131–144.
- Wang, Z.; Guo, H.M.; Liu, H.Y.; Zhang, W.M. Source, Migration, Distribution, Toxicological Effects and Remediation Technologies of Arsenic in Groundwater in China. China Geol. 2023, 6, 476–493.
- Wang, Y.X.; Li, J.X.; Ma, T.; Xie, X.J.; Deng, Y.M.; Gan, Y.Q. Genesis of Geogenic Contaminated Groundwater: As, F and I. Crit. Rev. Env. Sci. Tec. 2021, 51, 2895–2933.
- Sun, Y.; Zhou, J.L.; Yang, F.Y.; Ji, Y.Y.; Zeng, Y.Y. Distribution and Co-Enrichment Genesis of Arsenic, Fluorine and Iodine in Groundwater of the Oasis Belt in the Southern Margin of Tarim Basin. Earth Sci. Front. 2022, 29, 99–114.
- Guo, Q.; Guo, H.M.; Yang, Y.C.; Han, S.B.; Zhang, F.C. Hydrogeochemical Contrasts between Low and High Arsenic Groundwater and Its Implications for Arsenic Mobilization in Shallow Aquifers of the Northern Yinchuan Basin, P.R. China. J. Hydrol. 2014, 518, 464–476.
- Wang, Y.; Jiao, J.J.; Cherry, J.A. Occurrence and Geochemical Behavior of Arsenic in a Coastal Aquifer–Aquitard System of the Pearl River Delta, China. Sci. Total Environ. 2012, 427–428, 286–297.
- Berg, M.; Stengel, C.; Trang, P.; Hungviet, P.; Sampson, M.; Leng, M.; Samreth, S.; Fredericks, D. Magnitude of Arsenic Pollution in the Mekong and Red River Deltas—Cambodia and Vietnam. Sci. Total Environ. 2007, 372, 413–425.
- Litter, M.I.; Ingallinella, A.M.; Olmos, V.; Savio, M.; Difeo, G.; Botto, L.; Farfán Torres, E.M.; Taylor, S.; Frangie, S.; Herkovits, J.; et al. Arsenic in Argentina: Occurrence, Human Health, Legislation and Determination. Sci. Total Environ. 2019, 676, 756–766.
- Dilpazeer, F.; Munir, M.; Baloch, M.Y.J.; Shafiq, I.; Iqbal, J.; Saeed, M.; Abbas, M.M.; Shafique, S.; Aziz, K.H.H.; Mustafa, A.; et al. A Comprehensive Review of the Latest Advancements in Controlling Arsenic Contaminants in Groundwater. Water 2023, 15, 478.
- Kusimi, J.M.; Kusimi, B.A. The Hydrochemistry of Water Resources in Selected Mining Communities in Tarkwa. J. Geochem. Explor. 2012, 112, 252–261.
- Rowland, H.A.L.; Omoregie, E.O.; Millot, R.; Jimenez, C.; Mertens, J.; Baciu, C.; Hug, S.J.; Berg, M. Geochemistry and Arsenic Behaviour in Groundwater Resources of the Pannonian Basin (Hungary and Romania). Appl. Geochem. 2011, 26, 1–17.
- Dhillon, A.K. Arsenic Contamination of India’s Groundwater: A Review and Critical Analysis. In Arsenic Water Resources Contamination; Springer: Cham, Switzerland, 2020; pp. 177–205.
- Hamidian, A.H.; Razeghi, N.; Zhang, Y.; Yang, M. Spatial Distribution of Arsenic in Groundwater of Iran, a Review. J. Geochem. Explor. 2019, 201, 88–98.
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and Fluoride Contaminated Groundwaters: A Review of Current Technologies for Contaminants Removal. J. Environ. Manage. 2015, 162, 306–325.
- Tsuji, J.S.; Chang, E.T.; Gentry, P.R.; Clewell, H.J.; Boffetta, P.; Cohen, S.M. Dose-Response for Assessing the Cancer Risk of Inorganic Arsenic in Drinking Water: The Scientific Basis for Use of a Threshold Approach. Crit. Rev. Toxicol. 2019, 49, 36–84.
- Ali, W.; Mushtaq, N.; Javed, T.; Zhang, H.; Ali, K.; Rasool, A.; Farooqi, A. Vertical Mixing with Return Irrigation Water the Cause of Arsenic Enrichment in Groundwater of District Larkana Sindh, Pakistan. Environ. Pollut. 2019, 245, 77–88.
- Mushtaq, N.; Masood, N.; Khattak, J.A.; Hussain, I.; Khan, Q.; Farooqi, A. Health Risk Assessment and Source Identification of Groundwater Arsenic Contamination Using Agglomerative Hierarchical Cluster Analysis in Selected Sites from Upper Eastern Parts of Punjab Province, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 999–1018.
- Gómez, J.J.; Lillo, J.; Sahún, B. Naturally Occurring Arsenic in Groundwater and Identification of the Geochemical Sources in the Duero Cenozoic Basin, Spain. Environ. Geol. 2006, 50, 1151–1170.
- Haugen, E.A.; Jurgens, B.C.; Arroyo-Lopez, J.A.; Bennett, G.L. Groundwater Development Leads to Decreasing Arsenic Concentrations in the San Joaquin Valley, California. Sci. Total Environ. 2021, 771, 145223.
- Walkera, M.; Seiler, R.L.; Meinert, M. Effectiveness of Household Reverse-Osmosis Systems in a Western U.S. Region with High Arsenic in Groundwater. Sci. Total Environ. 2008, 389, 245–252.
- Medunić, G.; Fiket, Ž.; Ivanić, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 183–233. ISBN 9789811385872.
- Daniele, L. Distribution of Arsenic and Other Minor Trace Elements in the Groundwater of Ischia Island (Southern Italy). Environ. Geol. 2004, 46, 96–103.
- Sorg, T.J.; Chen, A.S.C.; Wang, L. Arsenic Species in Drinking Water Wells in the USA with High Arsenic Concentrations. Water Res. 2014, 48, 156–169.
- McClintock, T.R.; Chen, Y.; Bundschuh, J.; Oliver, J.T.; Navoni, J.; Olmos, V.; Lepori, E.V.; Ahsan, H.; Parvez, F. Arsenic Exposure in Latin America: Biomarkers, Risk Assessments and Related Health Effects. Sci. Total Environ. 2012, 429, 76–91.
- Ortega-Guerrero, A. Evaporative Concentration of Arsenic in Groundwater: Health and Environmental Implications, La Laguna Region, Mexico. Environ. Geochem. Health 2017, 39, 987–1003.
- Morales-Arredondo, J.I.; Esteller-Alberich, M.V.; Armienta Hernández, M.A.; Martínez-Florentino, T.A.K. Characterizing the Hydrogeochemistry of Two Low-Temperature Thermal Systems in Central Mexico. J. Geochem. Explor. 2018, 185, 93–104.
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O.; Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259-284, .
