Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The removal of sulfur- and nitrogen-containing compounds present in fuels is and will be crucial to accomplish actual strict regulations to avoid environmental and humanity health adversities. The conventional hydrodesulfurization and hydrodenitrogenation processes conducted by refineries are limited due to severe operating conditions, and even more importantly, they are inefficient for simultaneously removing nitrogen- and sulfur-containing compounds in fuels.
- denitrogenation
- desulfurization
- fuels
- acid rains
- oxidation
1. Introduction
Fossil fuels remain the main natural resource available to supply the enormous energy needs of current society. Furthermore, it is expected to comprise an impressive increment of energy consumption until 2040, associated with the expansion of the planet’s population by 1.7 billion, while it is anticipated that renewable energy will only have a 20% higher contribution than the actual by 2040 [1]. This scenario predicts that largely fossil fuels will be used to satisfy the lion’s share of global energy demand in the years ahead [2]. Fossil fuel usage compromises the health of our planet and humanity via pollution and large toxic gas emissions [3]. Heavy crude and fuel oils produced from unconventional sources are rich in sulfur- and nitrogen-containing compounds (SCCs and NCCs). Conventional crude oil produced from unconventional sources like oil shale, oil sand, vacuum residue, and coal are rich in SCCs and NCCs [4], whose removal demands immediate attention. Both sulfur and nitrogen have deleterious impacts on the environment as well as human health, and thus, limitations are imposed on their maximum permissible limits [5][6][7]. These are responsible for the acid rains that are associated with major nefarious health, cultural heritage, and environmental effects, further contributing to escalating climate instability. The refining industry has made an effort to remove nitrogen and sulfur compounds from crude oil, mainly to improve the adverse effects on the quality of the refined products and the negative environmental impact [8]. The presence of nitrogen compounds in crude oil reduces the efficiency of the industrial process of hydrodesulfurization (HDS) via competitive adsorption of the catalyst. NCCs can poison the catalytic converters, reducing fuel economy, and even acidify lubricating oils [9][10]. During the last decade, the use of biofuels has increased to diminish the use of fossil fuels, mainly in the transportation sector. Biofuels are produced from biological sources and considered to be cleaner and greener fuel. Their sulfur content is low, but the amount of NCCs which must be removed to promote biofuel commercialization is considerably high (12.4% in terms of relative % C content) [11][12]. The removal of SCCs and NCCs from fuel oils is essential for bettering the environment, our assets, human health, and refinery process development. Various studies on separately removing NCCs or SSCs from fuel oils can be found in the literature, with reasonable removal efficiency (Figure 1).
Figure 1. Schematic representation of the volume of isolated and simultaneous desulfurization and denitrogenation reported studies.
2. Extractive Process
Extractive desulfurization (EDS) and extractive denitrogenation (EDN) are energetically sustainable, cost-effective methods to produce low-sulfur and nitrogen fuels. These techniques can be performed simultaneously (EDS/N) and do not require the utilization of hydrogen or catalysts, as well as operating under simple conditions of low temperature and pressure [13][14]. Furthermore, these techniques are not destructive methods and thus do not have any impact on the quality of liquid fuels or on the chemical structure of the extracted valuable S- and N-compounds, which can be further processed into value-added products [15]. EDS and EDN are performed through a simple liquid–liquid extraction where the feedstock is mixed with an appropriate immiscible solvent in a biphasic system. S- and N-containing compounds present in the fuel oil are transferred to the appropriate solvent and separated by taking advantage of their nucleophilic properties and polarity (Figure 2).
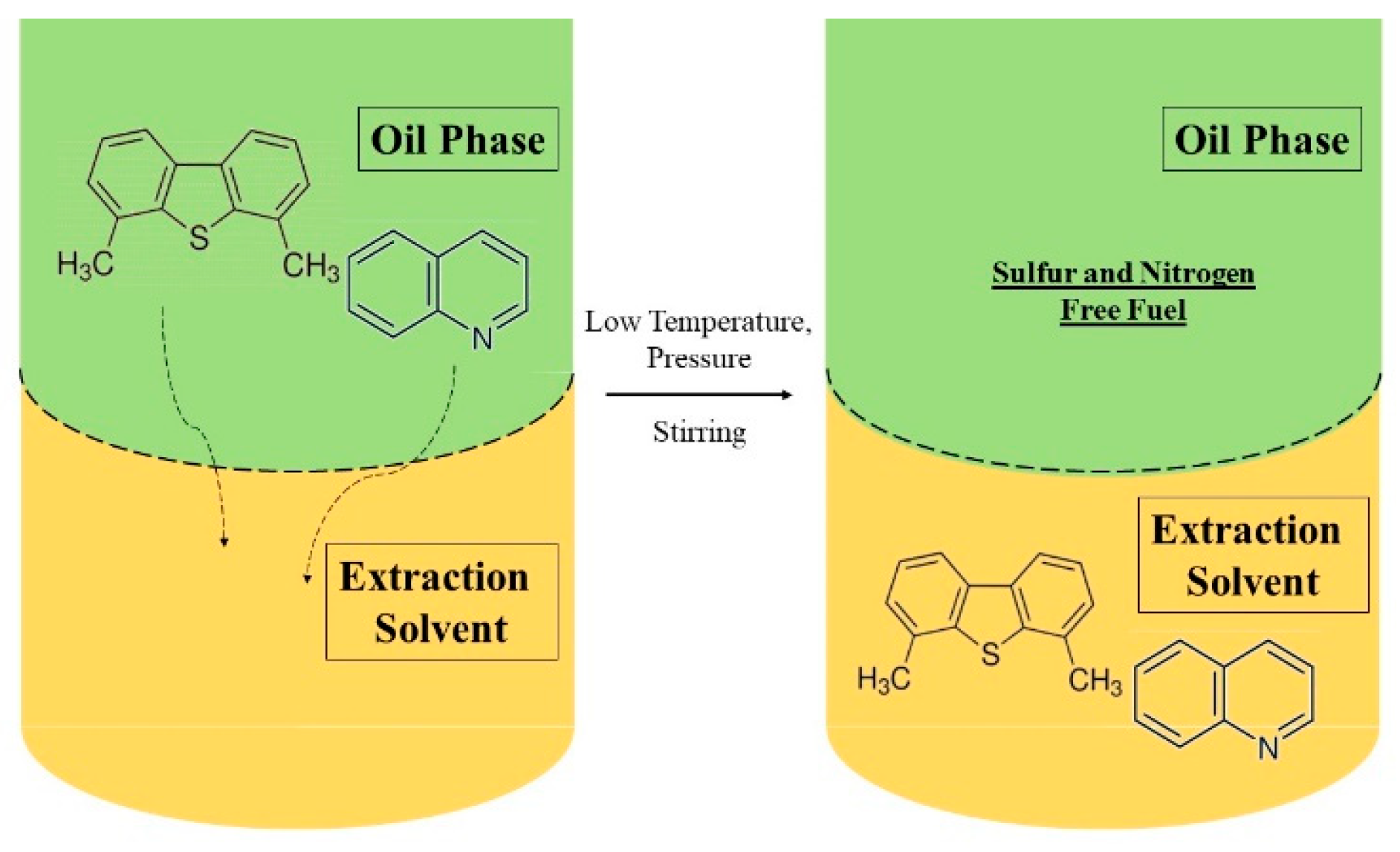
Figure 2. Schematic representation of the extractive process of a biphasic system of a fuel oil containing S and N compounds and an extraction solvent.
After the adequate extraction time (frequently only a few minutes, 10–30 min.), the immiscible extraction solvent is easily separated from the fuel oil, and the organosulfur and nitrogen compounds are then removed to recover the extraction solvent via distillation. Further, this can be recycled to perform multiple extraction cycles [13]. The choice of an adequate extraction solvent plays a crucial role in the efficiency of this method. Desirable properties for an effective solvent for simultaneous EDS/N must comprise a high partition coefficient for sulfur and/or nitrogen compounds, negligible solubility in fuels, high thermal and chemical stabilities, nontoxicity, and low costs.
2.1. Ionic Liquids
Ionic liquids (ILs) as extraction solvents are attracting the most attention in desulfurization literature, but also in EDS/N [16]. Several reviews of extractive desulfurization and extractive denitrogenation have been written [17][18][19]; however, a gap of information is found in the literature, conciliating both EDS/N processes. ILs comprise organic cations and either organic or inorganic anions (Figure 3) with 1018 possible combinations, granting them designations such as “task specific” or “designer solvents”, as their physicochemical properties can be tailored to specific applications [18].
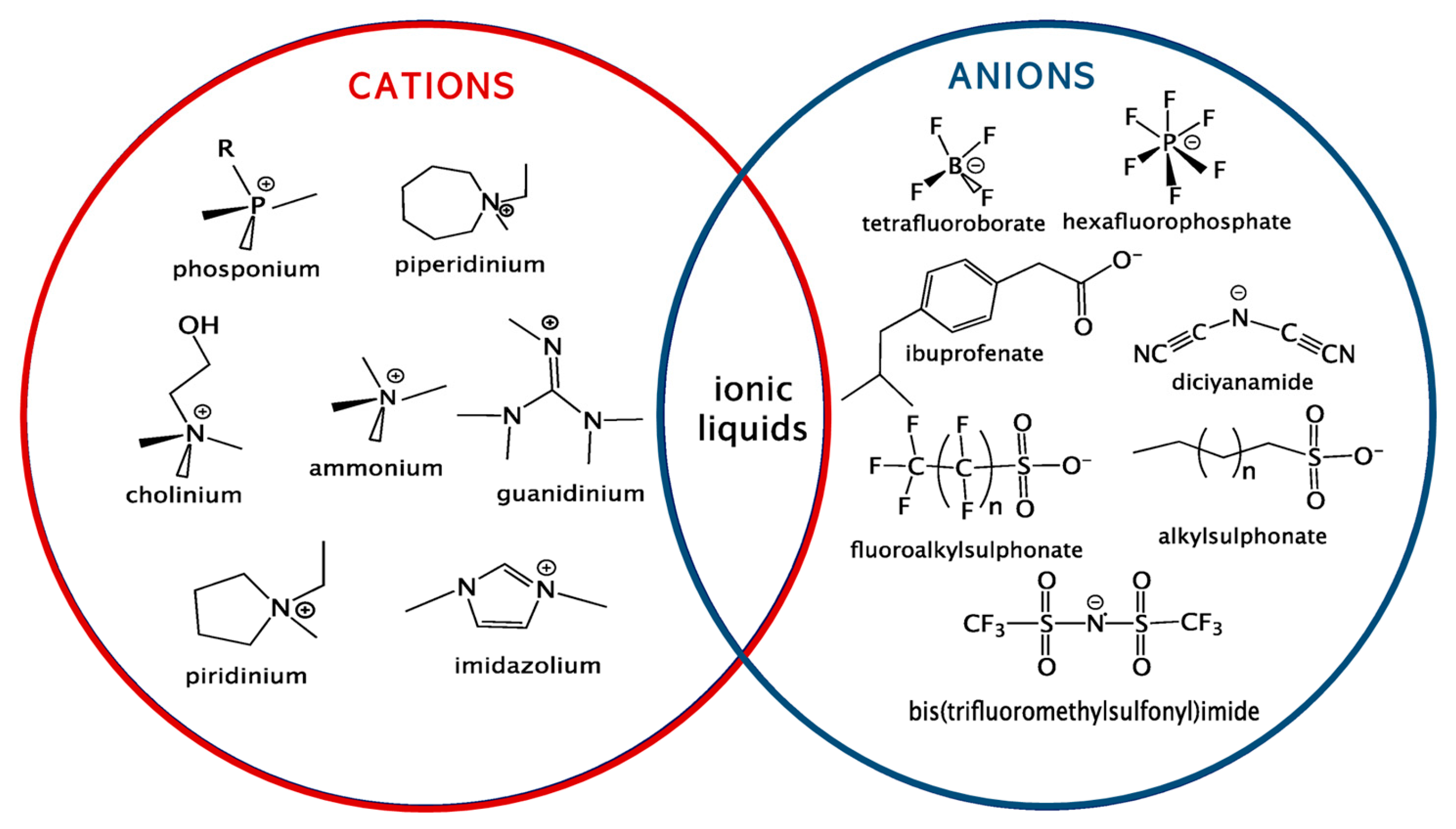
Figure 3. Representation of some of the most representative cations and anions present in ILs.
The use of ILs as extraction solvents for fuel desulfurization and denitrogenation comes from their generally negligible vapor pressure, which allows the extracted product to be further separated from the ILs. Several studies using ILs for simultaneous desulfurization and denitrogenation have been conducted, allowing researchers to establish key factors in their design. ILs should be completely insoluble in oil, very selective towards S- and N-compounds (avoiding harming the other properties of fuels), affordable, easy to regenerate, and thermally and chemically stable.
The pioneer study on the use of ILs for EDS/N of fuels reported different types of ILs, 1-alkyl-3-methylimidazolium (AMIM) tetrafluoroborate, hexafluorophosphate, and trimethylamine hydrochloride (AlCl3-TMAC) with potential to be applied for sulfur removal in transportation fuels [14]. At low concentrations, S- and N-compounds were successfully removed from the fuel oil without competing, while N-compounds, such as pyridine and 2-methylpyridine, were miscible with the IL BMIMPF6 (BMIM means 1-Butyl-3-methylimidazolium). However, at higher concentrations, it was found that performing N and S extraction was more difficult, although aromatic N-compound absorption was favored compared to S-compound extraction.
Hansmeier et al. studied pyridinium-based ILs as well as imidazolium-based ILs, and the two types of ILs were able to extract S- and N-containing heteroatoms from fuel oil systems [20]. ILs showed up to 50% more efficiency than other organic extraction solvents. It was possible to see that N-compounds were extracted more easily than S-compounds. Furthermore, it was also verified that for ILs to perform extractive processes, it was more important to have a higher capacity than a higher selectivity [21].
Chen et al. studied the extraction properties of Lewis acidic ILs 1-butyl-3-methylilimadazolium chloride/ZnCl2 and [BMIM]ZnCl2, as well as Brønsted acidic ILs [BMIM]HSO4 and [HMIM]SO4 (HMIM means 1-Hexyl-3-methylimidazolium) [13]. It was shown that [BMIM]ZnCl2 had better results, obtaining 93.8% of both thiophene and carbazole removal. Chen et al. also studied temperature influence on the extractive process and concluded that it did not play an important role on the efficacy of the IL. It was also shown that reducing the mass ratio between IL and oil did not affect N-compound removal but significantly decreased sulfur removal.
ILs present a higher extractive performance compared with other organic solvents; however, these can also be toxic, corrosive, and explosive. Further, IL synthesis is also far from being environmentally friendly, since it generally requires a large quantity of salts and solvents to achieve complete anion exchange, which also turns the price of these solvents extremely high [22][23][24][25].
Ongoing development of novel ILs with different constituents provides an opportunity for researchers to extend the application of these solvents; however, their undesirable aspects have led to the development of a selection for a new generation: low-cost ILs of facile preparation, deep eutectic solvents (DESs).
2.2. Deep Eutectic Solvents
DESs are a new generation of potential sustainable solvents, resulting from the eutectic mixture of green, easily available, and cheap raw components, capable of self-association [26]. These solvents exhibit physicochemical properties that are similar to those of traditionally used ILs while being inexpensive and environmentally friendly, as their preparation does not require expensive organic solvents. DESs have been applied in organic synthesis, electrochemistry, materials science, and catalysis [27][28][29][30]. Used in EDS/N, DESs should exhibit the same desirable properties as those expected for ILs. DESs should selectively remove sulfur and nitrogen compounds while avoiding co-extraction of other aromatic species from fuel oil and exhibit low miscibility with alkyl chains in fuel, reducing cross-contamination. To ensure optimal efficiency, DESs should keep their viscosity at a minimum, promoting efficient mass transfer of sulfur species between the two phases.
The first reported work about the extraction of S- and N-containing compounds from mixtures with n-heptane using tetrahexylammonium bromide-based DES solvents was performed by Alli and Kroon [31]. The S- and N-containing compounds separated from the raffinate phase were benzothiazole and thiophene. Through selectivity and distribution coefficient studies, it was possible to achieve benzothiazole with a greater affinity in comparison to thiophene. Lima et al., studied the capacity to remove S- and N-compounds from the fuel oil of four other different DESs by combining one ammonium- or phosphonium-based salt with one complexing agent (PEG400 and Sulfolane) [32]. It was shown that in simultaneous EDS/N, the removal of N-compounds was favored by the presence of concurrent S-compounds, and it was possible to achieve the same level of extractive desulfurization as a single EDS process. Moreover, reusability studies showed that DESs suffer a significant reduction in the efficiency of extraction for S-compounds but not for N-compounds. This setback was completely overcome after a regeneration process where the performance of DESs was fully restored [33].
The ability of DESs to perform simultaneous EDS/N from real fuel was studied by Warrag et al. using a phosphonium-based DES of methyl triphenylphosphonium bromide and triethylene glycol [34]. After nine cycles of extraction, it was possible to remove all the S- and N-compounds from the fuel oil, with each cycle being performed at 298 K for 1 h. While the quinoline (QUI) was removed after only two extraction cycles, the thiophene required more cycles. Lemaoui et al. also studied simultaneous desulfurization and denitrogenation of real fuels via liquid–liquid extraction, using acidic DES [35]. The utilization of a mixture of tetrapropylammonium bromide and acetic acid allowed them to achieve complete removal of pyrrole and pyridine in only two extraction stages, as well as achieving 89% removal of thiophene after five cycles of 1 h each at 298 K, showing an increase in desulfurization compared to the previous work from Warrag et al.
2.3. Other Solvents
Following the search for nonionic solvents able to perform simultaneous extractive desulfurization and denitrogenation, Zhu et al. [36] recently published a study about the application of polyether amine-based solvent. This was considered a green, low-cost, and efficient extraction solvent. The removal of S-containing compounds (72.16% for thiophene, 63.96% for dibenzothiophene, and 53.01% for benzothiophene) and N-containing compounds (98.31% for indole (IND), 97.12% for pyrrole, 91.51% for pyridine, and 89.93% for QUI) was achieved. After five cycles of extraction, the extractive capacity of this solvent was still maintained.
3. Adsorptive Process
Adsorptive denitrogenation (ADN) and desulfurization (ADS) are energetically sustainable methodologies for producing cleaner fuels. Effective materials able to conciliate ADS and ADS require an elaborative chemical structural designer to achieve physicochemical adsorption of organic S- and N-compounds. Most of the solid adsorbents present extensive surface areas, with suitable pore volumes containing appropriate active sites, and must contain structural robustness and stability. Further, these materials must be easily regenerated, allowing their use for consecutive ADN and ADS cycles under conditions of mild temperature and pressure [37]. The adsorptive process presents the advantage of absence of chemical reactions, ensuring the absence of impurities from by-products in fuels and safeguarding the physicochemical properties of treat fuels.
The adsorption of organosulfurs/organonitrogens can be explained through physical and/or chemical processes, namely Van der Waals (V. d. W.) interactions, Lewis acid–base interactions, and π complexation [38]. N-compounds can also be adsorbed through hydrogen bond formation with adequate surface functional groups [39]. The simplest of these interactions is Van der Waals, a simple physical adsorption process producing weak adsorbent–adsorbate interactions, especially in low-temperature applications [40]. Acid–base interactions are the most common chemical mechanism for S and N adsorption. This adsorption theory postulates that the coordinatively unsaturated metal acidic sites or functional groups in the adsorbent bind with the basic S or N moieties in the adsorbate through Lewis acid–base interactions [39]. π complexation comprises the transfer of electrons between π orbitals N/S-compounds and vacant metal sites in the adsorbent [41]. Understanding these adsorption mechanisms helps direct research towards the rational design of novel adsorbents featuring remarkable textural properties, adequate surface-active sites and defect density, robustness, and stability [42]. Recent research in the field of adsorbents for simultaneous ADS and ADN has focused on carbon-based materials, zeolites, mesoporous silica, and metal–organic frameworks (MOFs), as well as their respective structural tuning towards higher organosulfur and organonitrogen removal capacities.
3.1. Carbon-Based Materials
Carbon-based materials are currently the subject of outstanding interest in the development of novel materials due to their varying properties, associated with morphology and the high variety of functional groups than can be introduced [43]. Both micro- and nanoscale carbon allotropes demonstrate potential towards sustainable sulfur and nitrogen adsorption from liquid fuels [44][45]; thus, examples based on the use of activated carbons, graphene, and carbon nanotubes will now be discussed.
Activated carbons (ACs) have been widely used as adsorbents in liquid-phase adsorptions due to their high surface areas (typically 500–4000 m2/g) [46]. Large pore volumes can also be found [47]. Beyond their textural properties, the adsorption capabilities of ACs are heavily influenced by the presence of oxygen-containing functional groups. In 1999, Pradhan et al. oxidized ACs using HNO3, H2O2, and (NH4)2S2O8 at different temperatures. The authors noted that, despite a reduction in surface area for oxidized ACs, their oxygen contents could be enhanced nearly three times, increasing their adsorption capabilities. The positive effect of increasing surface oxygen functional groups compensates the negative effect of the decrease in microporosity under mild oxidation [48]. This work served as a basis for Ania et al. to oxidize an AC with (NH4)2S2O8, finding an increase of 74% in DBT adsorption compared to that of the original AC [49]. Li et al. also reported that the adsorption capacities of the basic N-compounds of an AC were effectively enhanced after a similar oxidation procedure [50]. However, Han et al. were the first authors to apply these ACs in simultaneous ADN/S, noting that treating the AC with 15 wt.% (NH4)2S2O8 increased N adsorption by 18% (from 0.40 mmol·g−1 to 0.47 mmol·g−1) but reduced S adsorption (from 0.18 mmol·g−1 to 0.16 mmol·g−1) capabilities [51]. The adsorption of simultaneous N- and S-compounds present in the liquid matrix follows distinct principles; while N adsorption is mostly dominated by the presence of oxygen from functional groups presented on the surface of ACs, the adsorption of S-compounds depends mostly on the textural aspects [51]. This suggests that ACs can be tailored to maximize adsorption of both classes of compounds simultaneously. Metal loading on ACs is another interesting route which aims to enhance their adsorption strength and selectivity and/or to introduce reactive adsorption routes via the incorporation of metallic species into the carbon matrix. Thaligari et al. prepared a Zn-impregnated granular AC and tested it as adsorptive material for DBT and QUI, demonstrating the competitive nature between the two species and noting that N-compounds are more favorably adsorbed than S-compounds [52]. A similar outcome was observed by Arcibar-Orozco when iron nanoparticles were introduced into an AC. In this case, a higher desulfurization and denitrogenation efficiency was observed when the processes were conducted separately. Performing simultaneous ADN/S, a reduction in sulfur adsorption was found using the modified AC. Furthermore, increasing the total nitrogen content present in the model fuel, the desulfurization capacity of the ACs decreased [53].
Nanoscale carbon allotropes have also been extensively explored for adsorption studies since these possess similar surface functionalities to ACs. Graphene is the building block for the graphitic materials of every other dimensionality, producing adsorbents with unique mechanical properties and extensive surface areas (theoretically as high as 2630 m2/g) [54], chemical inertness, and remarkable electrical and thermal conductivities [55]. Chemical treatment of graphite through oxidation, with subsequent dispersion and exfoliation in water or suitable organic solvents, produces graphene oxide (GO), a functionalized form of graphene, which is decorated with oxygen-containing functional groups [56]. Nanoscale carbons have been extensively studied as adsorptive materials for single ADS [57]; however, their application towards ADN seems to significantly lag behind. Li et al. prepared hollow mesoporous carbon nanospheres (HMCNs) that were further oxidized with HNO3, H2SO4, and (NH4)2S2O8 to introduce oxygen-containing groups and obtain stronger acidity, promoting acid–base interaction and hydrogen bonding between the adsorbents and QUI/IND molecules. Interestingly, the presence of sulfur compounds in the matrix did not significantly affect nitrogen compound adsorption performance, unlike what was previously observed for ACs. However, HMCNs also preferentially adsorb nitrogen over sulfur compounds (Figure 4) [58].
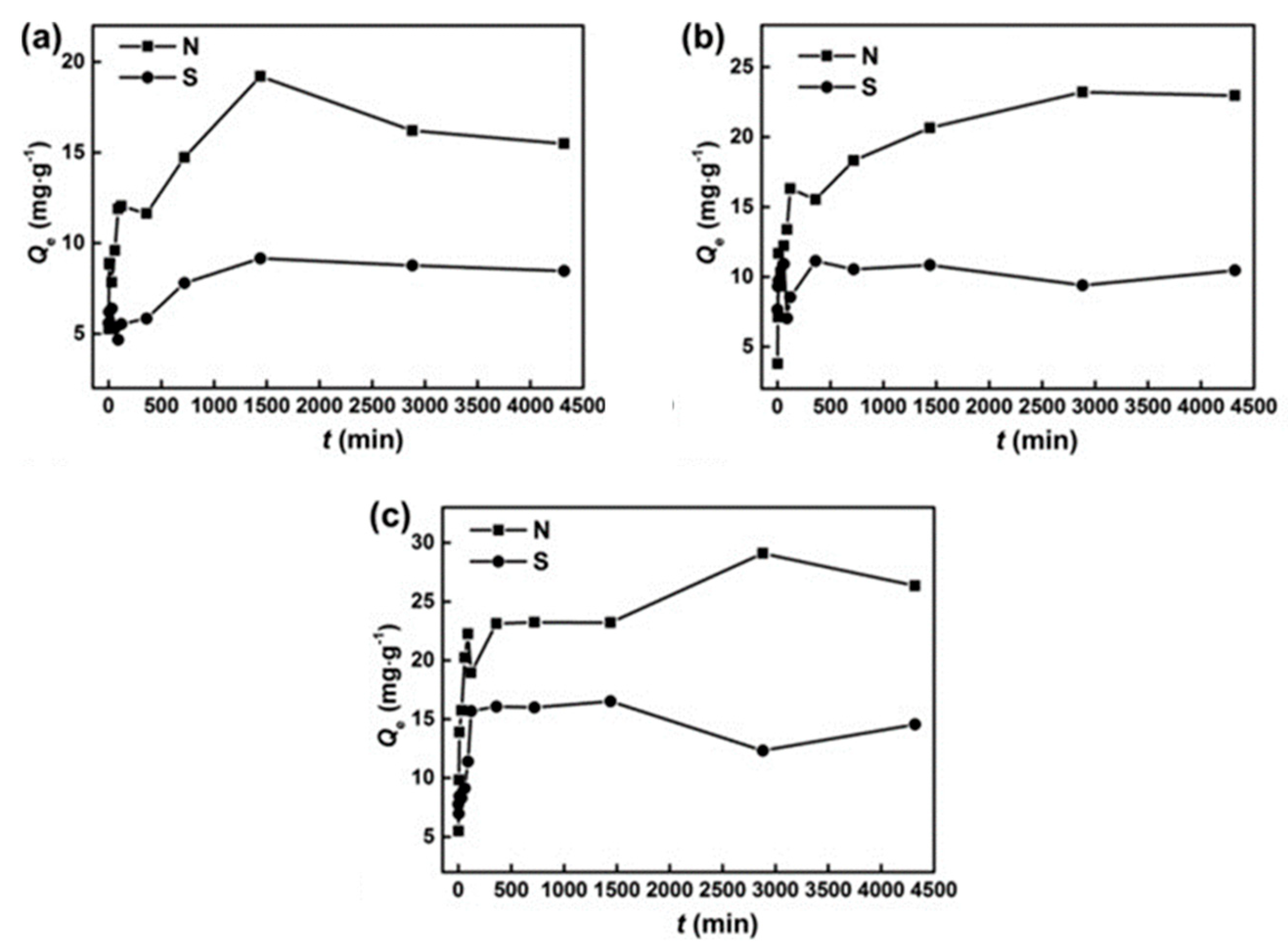
Figure 4. Adsorption curves of NCCs and SCCs over oxidized HMCNs at (a) 50 °C, (b) 100 °C, and (c) 150 °C.
The only work reporting simultaneous ADN/S with nanoscale carbon materials involved the formation of a graphene oxide (GO) composite and the MOF MIL-101 [59]. The composite displayed increased NCC adsorption capacities (~42% higher than the pristine MOF) due to the combined effect of the increase in porosity and the inclusion of hydrogen bonding capabilities from the added oxygen containing surface functional groups. When this adsorbent was applied in a model oil containing QUI, IND, and 1-BT, it was found that the presence of the SCC did not affect NCC adsorption. However, the adsorption of 1-BT was negligible [59], in accordance with previously mentioned studies for the simultaneous adsorption of NCCs and SCCs using carbon-based materials. In conclusion, studies using carbon materials, in general, will need further research towards the development of novel modification strategies in order to produce adsorbents with higher capacities to simultaneously remove NCC and SCC ADN/S.
3.2. Zeolites
Zeolites are microporous three-dimensional crystalline aluminosilicates with high external surface areas (150–500 m2/g) which are commonly used as adsorbents and catalysts. These types of materials are some of the most investigated for ADS due to their stable skeleton structure and different shapes (channels, cages, cavities, etc.) and sizes. Zeolites present remarkable thermal, mechanical, and thermal properties, as well as the ability to perform ion exchange on surface active sites, which significantly boosts their adsorption capacity and selectivity towards S- or N-compounds [60].
Synthetic zeolites are widely employed as adsorbents in a variety of adsorption processes, as careful control of the conditions of their processing allows for control of the structure and surface characteristics of the obtained adsorbent [61]. Plenty of research has been conducted on the use of zeolites for ADS processes, and a recent review by Dehghan et al. has effectively condensed the important parameters which influence the sulfur adsorption capacity for a given zeolite: texture properties, pore size, Si/Al ratio, the number of active surface sites, their acidic properties, and the charge of introduced metal cations [62]. The influence of each of these parameters has been confirmed through computational methods by Mguni et al., who developed a machine learning method to reach a consensus on the parameters with dominating influence on the S adsorption capabilities of a given zeolite. Beyond the previously mentioned parameters, this machine learning algorithm also identified the initial adsorbent concentration as the main parameter of adsorption efficiency [63]. As in the case of carbon-based materials, ADN research using zeolites significantly lags its ADS counterpart. Hernández-Maldonado et al. prepared a Y zeolite containing cuprous cations through ion-exchange procedures and tested its adsorption capacity for pyrrole, aniline, carbazole, and IND, comparing it with thiophene. The researchers found that, similarly to metal-doped carbon-based materials, CuY zeolite preferentially adsorbs organonitrogens. They suggest that, since adsorption follows the classical picture of π complexation, zeolites with other d-block cations are expected to preferentially adsorb the organonitrogen compounds as well, which is in accordance with the molecular orbital theory [64]. Zhang et al. had previously reported that a Cu-exchanged Y zeolite could adsorb ~50% more DBT than the pristine adsorbent, and the adsorption of SCCs using d-block metal exchanged Y zeolites mainly occurs through π complexation [65], which could explain the simultaneous deep ADN/S. Zhang et al. studied the effect of ion-exchanged Y zeolite with cerium [66] on ADN, since it was previously reported that the incorporation of La3+ species into aluminosilicate positively affected its sulfur adsorption capacity due to the introduction of the possibility for direct interactions between the lanthanum ions and the adsorbents [67][68]. The researchers found that increasing the amount of Ce in the adsorbent did not necessarily imply an increase in denitrogenation efficiency (Figure 5). Increasing Ce loading above the optimum value (7.46 wt.%), agglomeration of CeO2 particles occurred on the surface of the adsorbent, resulting in a decrease in adsorption properties. CeY zeolite achieved ADN efficiencies near two and two and a half times higher than its pristine counterpart for the adsorption of QUI and IND, respectively.
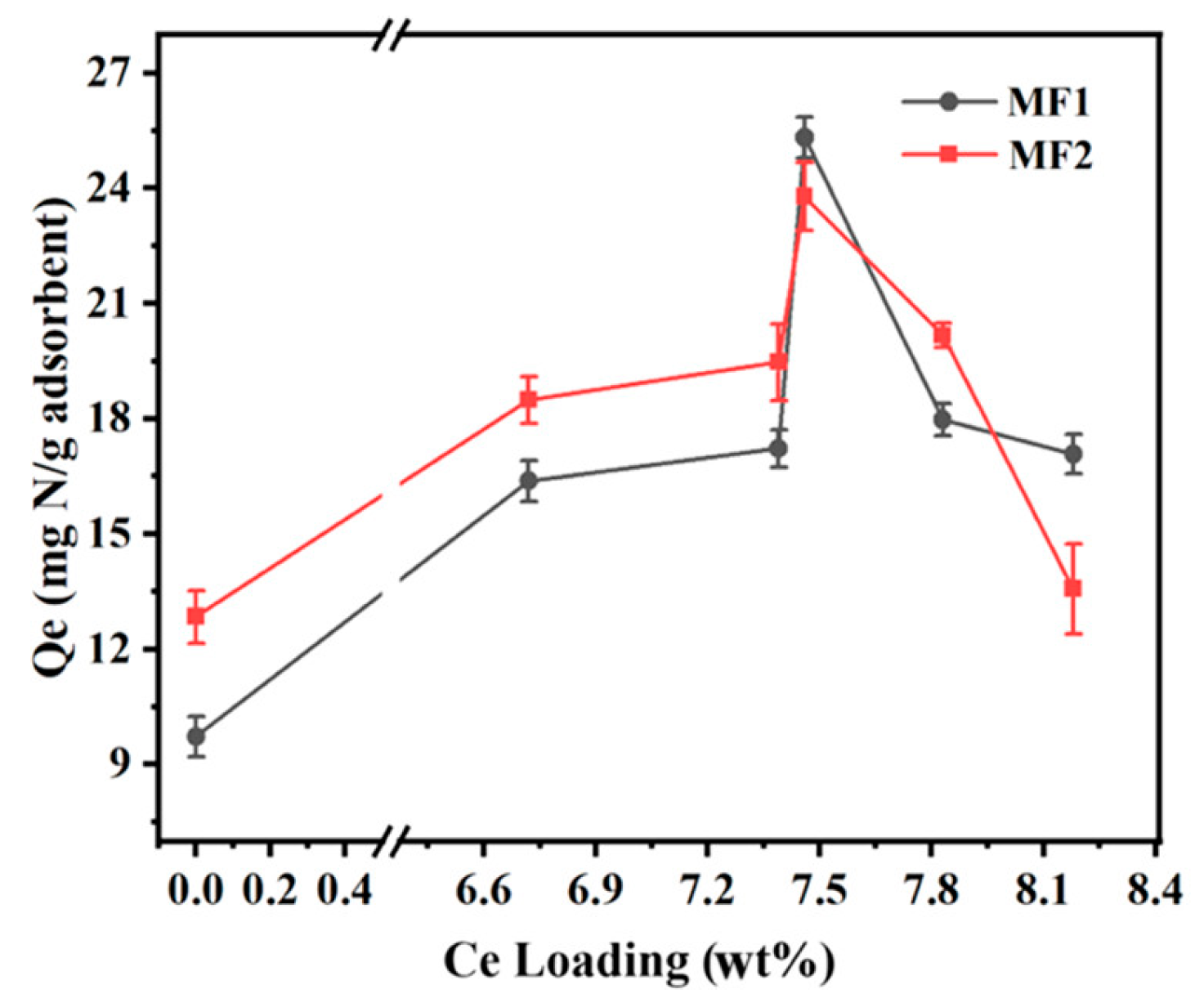
Figure 5. Effect of Ce loading on the N adsorption capability of Y zeolite for two different model fuels (200 ppm of QUI or IND for MF1 or MF2, respectively).
The QUI adsorption capacity of the spent CeY zeolite in its third cycle (14.01 mgN gA−1) was still higher than that of the original NaY adsorbent (9.72 mgN gA−1). This ion-exchanged zeolite was especially selective towards NCCs in the presence of naphthalene, a competitive aromatic component. The selectivity towards the adsorption of NCCs over other aromatic compounds was also demonstrated by Tian et al., who incorporated Yttrium into Y zeolite. This incorporation led to a decrease in BET surface area and pore volume, compensated for by the introduction of strong Brönsted acidity and medium Lewis acidity. When used as an adsorbent in a model fuel containing NCCs and toluene, its capacity to adsorb NCCs was maintained, unlike in the pristine zeolite, which lost near 10% adsorption capacity for QUI and near 30% for IND, demonstrating the importance of acid–base interactions in the adsorption selectivity of zeolites [69].
3.3. Mesoporous Silica
The large specific surface area (700–1300 m2/g) and pore volume (0.5–1.2 cm3/g), tunable pore size, and narrow pore distribution of mesoporous silica materials have granted a vested research interest in these materials for ADN/S [70]. Kwon et al. first tested the simultaneous adsorption capabilities of a spherical mesoporous silica (YSP-1) over treated (hydrodesulfurized fuel < 10 ppm N and <1900 ppm S) and untreated (190 ppm N and 8200 ppm S) fuels [71]. This silica-based adsorbent, with a surface area of 1331 m2·g−1 and an average pore size of 24 Å, adsorbed 8.14 mgN gA−1 from the untreated fuel, much higher than that for a previously reported silica–zirconia co-gel (3.71 mgN gA−1, SBET = 502 m2 g−1) [72]. This result demonstrates the importance of extensive surface areas for effective adsorption. Zirconia cations were introduced into the mesoporous structure of YSP-1, producing YSP-2 (average pore size and surface area of 31 Å and 1092 m2 g−1, respectively). YSP-2 adsorbs 29% more of S (7.74 mgS gA−1) than YSP-1 (5.99 mgS gA−1) while retaining similar N adsorption capacity, suggesting that metal incorporation in silicas is a viable strategy for producing simultaneous ADN/S adsorbents. Koriakin et al. took a similar approach, incorporating lithium into YSP silica and introducing adsorbate–metal interaction capabilities [73]. Compared to the original YSP, the YSP-Li possessed a smaller surface area (815.16 m2/g) and a larger pore size (3.73 nm). The adsorption capacity of this silica was compared to that of lithium impregnated in siliceous foam (Li-MCF) (551.59 m2/g and 19.62 nm surface area and pore size, respectively). YSP-Li adsorbed near 41% of NCCs, higher than what was observed for MCF-Li (25.9%). The removal percent for sulfur compounds was negligible for both adsorbents.
3.4. Metal–Organic Frameworks
MOFs are a class of porous 3D materials produced from the combination of multidentate organic linkers and metal ions/clusters. The huge number of possible linker/metal combinations ensures that multiple topologies can be produced, which are often accompanied by thermal, chemical, and mechanical resistance, extensive surface areas, and tailorable porosity [74]. These novel materials have garnered considerable interest in several areas of applications, with adsorption among them. In fact, the potential of MOFs for ADS has already been covered in a recent review by Saha et al. [75] Thus, this research will now focus on their application in ADN and simultaneous ADN/S. One of the first and most thorough investigations into the potential of MOFs for simultaneous ADN/S was reported by Maes et al. in 2011. The researchers compared the adsorptive capacity of various MOFs: MIL-100 with different metal centers (Fe, Cr, Al), MIL-101(Cr), MIL-53, HKUST-1, and CPO-27 with either Ni or Co. They found that MOFs with open metal sites (i.e., all studied MOFs except MIL-53) display a significantly higher uptake of both NCCs and SCCs than those without open metal sites (i.e., MIL-53). Furthermore, the selectivity of MOFs containing Al3+, Cr3+, and Fe3+ for NCCs was demonstrated and explained through Pearson’s concept of hard/soft acid/bases (hard N bases prefer interactions with hard Lewis’s acid sites). In fact, the selectivity and remarkable adsorption capacity of MIL-101 for NCCs from fuels had already been reported by Nuzhdin et al., producing an adsorbent with one of the highest N uptakes to the date of publishing [76]. Following a comparative research line, Jhung et al., attempted to understand the effect of the acidity or basicity of MOFs on their ADN capabilities. MIL-100(Cr) was modified to impart acidity or basicity by grafting ethylenediamine (ED) and aminomethane sulfonic acid (AMSA) onto its coordinatively unsaturated sites. Despite a decrease in BET surface area (from 1462 m2/g to 1351 m2/g), the acid-functionalized MOF (AMSA-MIL-100) displayed an effective increase in the adsorptive removal of QUI (from 160 mg·gA−1 to 190 mg·gA−1) and BT (from 18 mg·gA−1 to 23 mg·gA−1) due to the introduction of acid–base interaction functionality. The introduction of basic functionalities (ED-MIL-100) decreased the adsorption performance of the MOF due to base–base repulsion with the basic adsorbates. Interestingly, functionalization of the MOF with either acid or basic groups decreased its IND adsorption capabilities, as this neutral NCC was preferentially adsorbed by Van der Waals interactions; thus, higher surface areas were the determining factor for its adsorption [77]. Ahmed et al. imbued MIL-125 with H-bonding capabilities by modifying its linker with −NH2 functional groups. The researchers verified that the adsorbed quantities of both IND and QUI increased in line with increasing the content of the −NH2 group in the MOF [78]. Mondol et al. took this approach a step further by introducing amino groups on both the ligand and metal sites of MIL-101(Cr). This functionalization caused a decrease in BET surface area (from 2488 m2/g to 1960 m2/g) but significantly increased IND adsorption while decreasing QUI removal, similar to the previously discussed research study. The MOF was further modified by the introduction of carboxylic groups (Figure 6), which boosted its NCC adsorption capacity to nearly double that of the pristine structure and about 10 times as much as that of a commercial AC, due to the introduction of H-bonding functionality in the adsorbent [79].
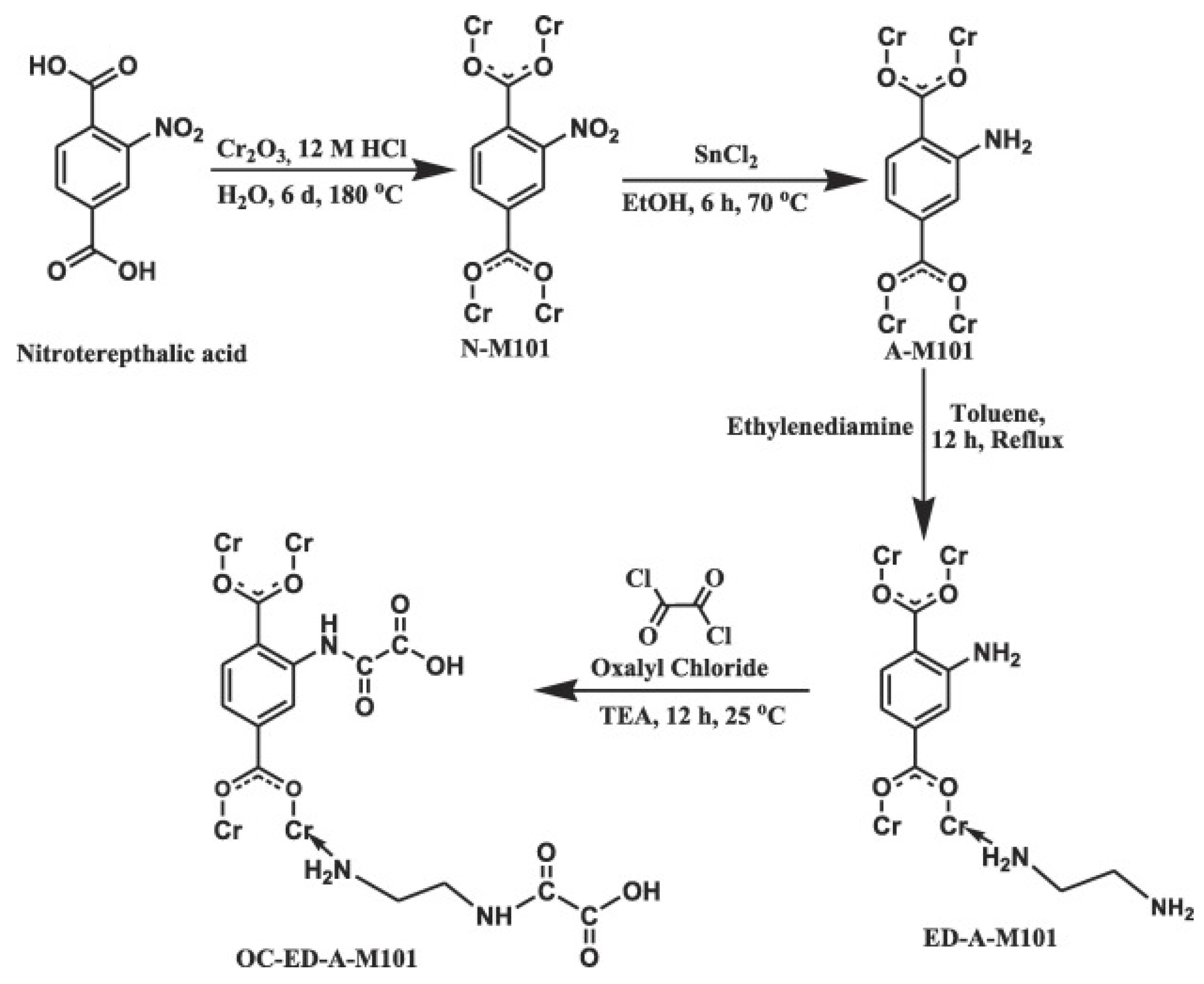
Figure 6. Representation of reaction route to prepare a difunctionalized MIL-101 derivative.
This entry is adapted from the peer-reviewed paper 10.3390/catal14020137
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792.
- Council, E.-A.B. Energy Transition in ASEAN 2023. 2023. Available online: https://www.eu-asean.eu/wp-content/uploads/2023/04/Energy-Transition-in-ASEAN-2023_5-April-2023.pdf (accessed on 4 April 2023).
- Shindell, D.; Smith, C.J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 2019, 573, 408–411.
- Hegazi, A.H.; El-Gayar, M.S. Role of non-hydrocarbon constituents in crude oils correlation and heavy fractions processing studies. Pet. Chem. 2017, 57, 838–842.
- Doney, S.C.; Mahowald, N.; Lima, I.; Feely, R.A.; Mackenzie, F.T.; Lamarque, J.F.; Rasch, P.J. Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proc. Natl. Acad. Sci. USA 2007, 104, 14580–14585.
- Sun, Y.X.; Zwolinska, E.; Chmielewski, A.G. Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 119–142.
- Syri, S.; Fronzek, S.; Karvosenoja, N.; Forsius, M. Sulphur and nitrogen oxides emissions in Europe and deposition in Finland during the 21st century. Boreal Environ. Res. 2004, 9, 185–198.
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631.
- Bello, S.S.; Wang, C.; Zhang, M.J.; Gao, H.; Han, Z.N.; Shi, L.; Su, F.B.; Xu, G.W. A Review on the Reaction Mechanism of Hydrodesulfurization and Hydrodenitrogenation in Heavy Oil Upgrading. Energy Fuels 2021, 35, 10998–11016.
- Drahoradova, A.; Vit, Z.; Zdrazil, M. Carbon Supported Ni-Mo Catalyst—High Hydrodenitrogenation Activity and Low Inhibition of Hydrodesulfurization by Hydrodenitrogenation. Fuel 1992, 71, 455–458.
- Miranda, A.M.; Ocampo, D.; Vargas, G.J.; Ríos, L.A.; Sáez, A.A. Nitrogen content reduction on scenedesmus obliquus biomass used to produce biocrude by hydrothermal liquefaction. Fuel 2021, 305, 121592.
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481.
- Chen, X.; Yuan, S.; Abdeltawab, A.A.; Al-Deyab, S.S.; Zhang, J.; Yu, L.; Yu, G. Extractive desulfurization and denitrogenation of fuels using functional acidic ionic liquids. Sep. Purif. Technol. 2014, 133, 187–193.
- Zhang, S.; Zhang, Q.; Zhang, C.Z. Extractive Desulfurization and Denitrogenation of Fuels Using Ionic. Ind. Eng. Chem. Res. 2004, 43, 614–622.
- Kianpour, E.; Azizian, S. Polyethylene glycol as a green solvent for effective extractive desulfurization of liquid fuel at ambient conditions. Fuel 2014, 137, 36–40.
- Rogers, R.D.; Seddon, K.R. Ionic Liquids--Solvents of the Future? Science 2003, 302, 792–793.
- Abro, R.; Abro, M.; Gao, S.R.; Bhutto, A.W.; Ali, Z.M.; Shah, A.; Chen, X.C.; Yu, G.R. Extractive denitrogenation of fuel oils using ionic liquids: A review. Rsc Adv. 2016, 6, 93932–93946.
- Paucar, N.E.; Kiggins, P.; Blad, B.; De Jesus, K.; Afrin, F.; Pashikanti, S.; Sharma, K. Ionic liquids for the removal of sulfur and nitrogen compounds in fuels: A review. Environ. Chem. Lett. 2021, 19, 1205–1228.
- Zolotareva, D.; Zazybin, A.; Rafikova, K.; Dembitsky, V.M.; Dauletbakov, A.; Yu, V. Ionic liquids assisted desulfurization and denitrogenation of fuels. Vietnam. J. Chem. 2019, 57, 133–163.
- Hansmeier, A.R.; Meindersma, G.W.; de Haan, A.B. Desulfurization and denitrogenation of gasoline and diesel fuels by means of ionic liquids. Green Chem. 2011, 13, 1907–1913.
- Meindersma, W.; De Haan, A.B. Separation Processes with Ionic Liquids. In Ionic Liquids Uncoiled; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 119–179.
- Kumar, M.; Trivedi, N.; Reddy, C.R.K.; Jha, B. Toxic Effects of Imidazolium Ionic Liquids on the Green Seaweed Ulva lactuca: Oxidative Stress and DNA Damage. Chem. Res. Toxicol. 2011, 24, 1882–1890.
- Uerdingen, M.; Treber, C.; Balser, M.; Schmitt, G.; Werner, C. Corrosion behaviour of ionic liquids. Green Chem. 2005, 7, 321–325.
- Wang, B.; Feng, Y.; Qi, X.; Deng, M.; Tian, J.; Zhang, Q. Designing Explosive Poly(Ionic Liquid)s as Novel Energetic Polymers. Chem. A Eur. J. 2018, 24, 15897–15902.
- Martyn, E.; Jose, E.; Manuela, G.; Luis, R.; Kenneth, S.; Joe, M.; Jason, W. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439, 831–834.
- Kongpol, K.; Chaihao, P.; Shuapan, P.; Kongduk, P.; Chunglok, W.; Yusakul, G. Therapeutic hydrophobic deep eutectic solvents of menthol and fatty acid for enhancing anti-inflammation effects of curcuminoids and curcumin on RAW264.7 murine macrophage cells. RSC Adv. 2022, 12, 17443–17453.
- Handy, S.; Lavender, K. Organic synthesis in deep eutectic solvents: Paal–Knorr reactions. Tetrahedron Lett. 2013, 54, 4377–4379.
- Nkuku, C.A.; LeSuer, R.J. Electrochemistry in Deep Eutectic Solvents. J. Phys. Chem. B 2007, 111, 13271–13277.
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50.
- Wang, Q.; Yao, X.; Geng, Y.; Zhou, Q.; Lu, X.; Zhang, S. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate)(PET). Green Chem. 2015, 17, 2473–2479.
- Alli, R.D.; Kroon, M.C. Extraction of benzothiazole and thiophene from their mixtures with n-heptane using tetrahexylammonium bromide-based deep eutectic solvents as extractive denitrogenation and desulfurization agents. Fluid Phase Equilibria 2018, 477, 1–11.
- Lima, F.; Dave, M.; Silvestre, A.J.D.; Branco, L.C.; Marrucho, I.M. Concurrent Desulfurization and Denitrogenation of Fuels Using Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 11341–11349.
- Prado, G.H.C.; Rao, Y.; de Klerk, A. Nitrogen Removal from Oil: A Review. Energy Fuels 2017, 31, 14–36.
- Warrag, S.E.E.; Darwish, A.S.; Abuhatab, F.O.S.; Adeyemi, I.A.; Kroon, M.C.; AlNashef, I.M. Combined Extractive Dearomatization, Desulfurization, and Denitrogenation of Oil Fuels Using Deep Eutectic Solvents: A Parametric Study. Ind. Eng. Chem. Res. 2020, 59, 11723–11733.
- Lemaoui, T.; Benguerba, Y.; Darwish, A.S.; Abu Hatab, F.; Warrag, S.E.E.; Kroon, M.C.; Alnashef, I.M. Simultaneous dearomatization, desulfurization, and denitrogenation of diesel fuels using acidic deep eutectic solvents as extractive agents: A parametric study. Sep. Purif. Technol. 2021, 256, 117861.
- Zhu, S.; Cheng, H.L.; Dai, Y.F.; Gao, J.J.; Jiang, X.M. Extractive Desulfurization and Denitrogenation from Fuel Oil by a Polyether-Amine-Based Solvent. Energy Fuels 2020, 34, 8186–8194.
- Chen, K.; Li, W.; Biney, B.W.; Li, Z.; Shen, J.; Wang, Z. Evaluation of adsorptive desulfurization performance and economic applicability comparison of activated carbons prepared from various carbon sources. RSC Adv. 2020, 10, 40329–40340.
- Duan, L.; Gao, X.; Meng, X.; Zhang, H.; Wang, Q.; Qin, Y.; Zhang, X.; Song, L. Adsorption, Co-adsorption, and Reactions of Sulfur Compounds, Aromatics, Olefins over Ce-Exchanged Y Zeolite. J. Phys. Chem. C 2012, 116, 25748–25756.
- Ahmed, I.; Jhung, S.H. Effective adsorptive removal of indole from model fuel using a metal-organic framework functionalized with amino groups. J. Hazard. Mater. 2015, 283, 544–550.
- Rangarajan, S.; Mavrikakis, M. DFT Insights into the Competitive Adsorption of Sulfur- and Nitrogen-Containing Compounds and Hydrocarbons on Co-Promoted Molybdenum Sulfide Catalysts. ACS Catal. 2016, 6, 2904–2917.
- Khan, N.A.; Uddin, N.; Choi, C.H.; Jhung, S.H. Adsorptive Denitrogenation of Model Fuel with CuCl-Loaded Adsorbents: Contribution of Π-Complexation and Direct Interaction between Adsorbates and Cuprous Ions. J. Phys. Chem. C 2017, 121, 11601–11608.
- Suib, S.L. A Review of Recent Developments of Mesoporous Materials. Chem. Rec. 2017, 17, 1169–1183.
- Kolahdouz, M.; Xu, B.; Nasiri, A.F.; Fathollahzadeh, M.; Manian, M.; Aghababa, H.; Wu, Y.; Radamson, H.H. Carbon-Related Materials: Graphene and Carbon Nanotubes in Semiconductor Applications and Design. Micromachines 2022, 13, 1257.
- Sikarwar, P.; Gosu, V.; Palla, V.C.S.; Subbaramaiah, V. Central composite design approach for concurrent desulfurization and denitrogenation of model liquid fuel over Mo-AAC. Environ. Qual. Manag. 2023.
- Zhu, J.; Yu, J.T.; Wu, P.W.; Liu, J.X.; Ji, H.Y.; Huang, Y.; Chao, Y.H.; Liu, H.Y.; Zhu, W.S.; Liu, Z.C. 3D printing of hierarchically porous lightweight activated carbon/alumina monolithic adsorbent for adsorptive desulfurization of hydrogenated diesel. Sep. Purif. Technol. 2024, 330, 125334.
- Li, S.; Han, K.; Li, J.; Li, M.; Lu, C. Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous Mesoporous Mater. 2017, 243, 291–300.
- Zhou, A.; Ma, X.; Song, C. Liquid-Phase Adsorption of Multi-Ring Thiophenic Sulfur Compounds on Carbon Materials with Different Surface Properties. J. Phys. Chem. B 2006, 110, 4699–4707.
- Pradhan, B.K.; Sandle, N.K. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 1999, 37, 1323–1332.
- Ania, C.O.; Bandosz, T.J. Importance of structural and chemical heterogeneity of activated carbon surfaces for adsorption of dibenzothiophene. Langmuir 2005, 21, 7752–7759.
- Li, N.; Zhu, J.; Ma, X.; Zha, Q.; Song, C. Tailoring of surface oxygen-containing functional groups and their effect on adsorptive denitrogenation of liquid hydrocarbons over activated carbon. AIChE J. 2013, 59, 1236–1244.
- Han, X.; Lin, H.; Zheng, Y. Adsorptive denitrogenation and desulfurization of diesel using activated carbons oxidized by (NH4)2S2O8 under mild conditions. Can. J. Chem. Eng. 2015, 93, 538–548.
- Thaligari, S.K.; Srivastava, V.C.; Prasad, B. Simultaneous Adsorptive Desulfurization and Denitrogenation by Zinc Loaded Activated Carbon: Optimization of Parameters. Pet. Sci. Technol. 2015, 33, 1667–1675.
- Arcibar-Orozco, J.A.; Rangel-Mendez, J.R. Model diesel denitrogenation by modified activated carbon with iron nanoparticles: Sulfur compounds effect. Chem. Eng. J. 2013, 230, 439–446.
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60.
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; khan, A.U. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163.
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053.
- Svinterikos, E.; Zuburtikudis, I.; Al-Marzouqi, M. Carbon Nanomaterials for the Adsorptive Desulfurization of Fuels. J. Nanotechnol. 2019, 2019, 2809867.
- Li, Z.; Liang, H.; Li, X.; Yang, C.; Ge, B.; Xiong, S.; Zhang, H.; Wang, T.; Yuan, P. Adjusting surface acidity of hollow mesoporous carbon nanospheres for enhanced adsorptive denitrogenation of fuels. Chem. Eng. Sci. 2020, 228, 115963.
- Ahmed, I.; Jhung, S.H. Remarkable adsorptive removal of nitrogen-containing compounds from a model fuel by a graphene oxide/MIL-101 composite through a combined effect of improved porosity and hydrogen bonding. J. Hazard. Mater. 2016, 314, 318–325.
- Jiang, K.; Li, Z.; Zheng, Z.; Li, J.; Qi, X.; Zhou, J.; Wei, H.; He, Y.; Xue, M.; Chu, H. Enhanced adsorption performance for aromatic sulfur compounds over a hierarchical structured AgX zeolite. Environ. Sci. Atmos. 2021, 1, 569–576.
- Zhao, X. 17—Porous materials for direct and indirect evaporative cooling in buildings. In Materials for Energy Efficiency and Thermal Comfort in Buildings; Hall, M.R., Ed.; Woodhead Publishing: Sawston, UK, 2010.
- Dehghan, R.; Anbia, M. Zeolites for adsorptive desulfurization from fuels: A review. Fuel Process. Technol. 2017, 167, 99–116.
- Mguni, L.L.; Ndhlovu, A.; Liu, X.; Hildebrandt, D.; Yao, Y. Insight into Adsorptive Desulfurization by Zeolites: A Machine Learning Exploration. Energy Fuels 2022, 36, 4427–4438.
- Hernández-Maldonado, A.J.; Yang, R.T. Denitrogenation of Transportation Fuels by Zeolites at Ambient Temperature and Pressure. Angew. Chem. Int. Ed. 2004, 43, 1004–1006.
- Zhang, Z.Y.; Shi, T.B.; Jia, C.Z.; Ji, W.J.; Chen, Y.; He, M.Y. Adsorptive removal of aromatic organosulfur compounds over the modified Na-Y zeolites. Appl. Catal. B Environ. 2008, 82, 1–10.
- Zhang, J.; Huang, L.; Lin, X.; Wang, Y.; Yu, Y.; Qi, T. Effective Adsorptive Denitrogenation from Model Fuels over CeY Zeolite. Ind. Eng. Chem. Res. 2022, 61, 14586–14597.
- Velu, S.; Ma, X.; Song, C. Selective Adsorption for Removing Sulfur from Jet Fuel over Zeolite-Based Adsorbents. Ind. Eng. Chem. Res. 2003, 42, 5293–5304.
- Xue, M.; Chitrakar, R.; Sakane, K.; Hirotsu, T.; Ooi, K.; Yoshimura, Y.; Toba, M.; Feng, Q. Preparation of cerium-loaded Y-zeolites for removal of organic sulfur compounds from hydrodesulfurizated gasoline and diesel oil. J. Colloid Interface Sci. 2006, 298, 535–542.
- Tian, F.; Sun, X.; Liu, X.; Zhang, H.; Liu, J.; Guo, H.; Zhang, Y.; Meng, C. Effective adsorptive denitrogenation from model fuels over yttrium ion-exchanged Y zeolite. Chin. J. Chem. Eng. 2020, 28, 414–419.
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118.
- Kwon, J.-M.; Moon, J.-H.; Bae, Y.-S.; Lee, D.-G.; Sohn, H.-C.; Lee, C.-H. Adsorptive Desulfurization and Denitrogenation of Refinery Fuels Using Mesoporous Silica Adsorbents. ChemSusChem 2008, 1, 307–309.
- Bae, Y.-S.; Kim, M.-B.; Lee, H.-J.; Lee, C.-H.; Wook Ryu, J. Adsorptive denitrogenation of light gas oil by silica-zirconia cogel. AIChE J. 2006, 52, 510–521.
- Koriakin, A.; Ponvel, K.M.; Lee, C.-H. Denitrogenation of raw diesel fuel by lithium-modified mesoporous silica. Chem. Eng. J. 2010, 162, 649–655.
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674.
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2021, 214, 106685.
- Nuzhdin, A.L.; Kovalenko, K.A.; Dybtsev, D.N.; Bukhtiyarova, G.A. Removal of nitrogen compounds from liquid hydrocarbon streams by selective sorption on metal-organic framework MIL-101. Mendeleev Commun. 2010, 20, 57–58.
- Ahmed, I.; Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive denitrogenation of model fuels with porous metal-organic frameworks (MOFs): Effect of acidity and basicity of MOFs. Appl. Catal. B Environ. 2013, 129, 123–129.
- Ahmed, I.; Khan, N.A.; Yoon, J.W.; Chang, J.-S.; Jhung, S.H. Protonated MIL-125-NH2: Remarkable Adsorbent for the Removal of Quinoline and Indole from Liquid Fuel. ACS Appl. Mater. Interfaces 2017, 9, 20938–20946.
- Mondol, M.M.H.; Bhadra, B.N.; Park, J.M.; Jhung, S.H. A remarkable adsorbent for removal of nitrogenous compounds from fuel: A metal–organic framework functionalized both on metal and ligand. Chem. Eng. J. 2021, 404, 126491.
This entry is offline, you can click here to edit this entry!
