Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
The development of severe multidrug-resistant bacterial infections has recently intensified because of the COVID-19 pandemic. According to the guidelines issued by the World Health Organization (WHO), routine antibiotic administration is not recommended for patients with supposed or confirmed mild SARS-CoV-2 infection or pneumonia, unless bacterial infection is clinically suspected.
- antibiotic resistance
- vaccine
- monoclonal antibody
1. Escalating Challenge of Antimicrobial Resistance in the Post-COVID Era
The global issue of antimicrobial resistance has been escalating rapidly. Each year, drug-resistant bacterial infections claim approximately 23,000 lives in the United States and 33,100 in Europe [1,2]. By 2050, the annual global death toll from multidrug-resistant (MDR) bacterial infections is projected to escalate to a staggering 10 million people [3]. A recent meta-study revealed that while nearly three-quarters of patients with SARS-CoV-2 were prescribed prophylactic antibiotics, only a mere 8.6% had confirmed bacterial co-infections (95% Confidence Interval 4.7–15.2%). This inappropriate and continuous antibiotic prescription practice significantly contributes to the rise in antibiotic resistance [4]. In the aftermath of the COVID-19 pandemic peak in 2020, the U.S. Centers for Disease Control and Prevention (CDC) reported a substantial increase in antibiotic use [5]. Consequently, the proportion of hospital-acquired antimicrobial-resistant microbial infections rose by 15%, including common pathogens such as MRSA (13%), VRE (14%), MDR P. aeruginosa (32%), and carbapenem-resistant Acinetobacter (78%) 5. These bacterial strains were responsible for 73.4% of all attributable deaths [6]. Despite the World Health Organization (WHO) and various national health agencies advocating for and establishing treatment guidelines during the COVID-19 pandemic, the overuse of antibiotics persists as a grave concern. This has intensified the silent pandemic of MDR bacteria [7], leading to increased mortality rates, prolonged hospital stays, and escalating medical costs. This poses a significant threat to both public health and national economies [1,8].
2. Developmental Challenges in Bacterial Vaccines
From the discovery and development of human medicines to the recent experience with COVID-19, vaccines have proven to be the most cost-effective strategies for the prevention of infectious diseases, even in immunocompromised populations [14]. Several studies have demonstrated that vaccinating against MDR bacteria is cost-effective, particularly in children aged 5 years or younger, as well as in lower middle-income and low-income countries where the burden of infectious diseases is relatively higher [15,16]. A recent study also estimated that pneumococcal conjugate vaccine (PCV13) reduced antibiotic-nonsusceptible invasive pneumococcal disease from 61% to 27% across all age groups in the U.S. [17]. Other studies have compellingly demonstrated typhoid conjugate vaccines (TCVs) as effective in reducing Salmonella typhi transmission in low-income countries. The World Health Organization (WHO) systematically confirms the TCV’s effectiveness in preventing typhoid fever spread in endemic regions, endorsing its inclusion in routine immunization programs, particularly in high-risk countries [18]. While antibiotics are gradually losing efficacy, there is a heightened urgency to develop new treatment strategies to combat the ever-changing MDR bacterial strains [19]. To highlight the urgency in the development of new therapeutic strategies, the focus on vaccines has shifted from being a mere topic of discussion to investigating their feasibility in clinical applications [20,21]. In multipopulational models, vaccination can inhibit resistance if it has a larger impact on subpopulations that consume more antibiotics [22]. However, three major challenges exist as hurdles in the development of MDR bacterial vaccines, viz., technical aspects, applicable groups, and economic considerations [23].
First, in technical identification of a suitable vaccine candidate are subunits from previous methods of utilizing virulence factors, surface sugar molecules, or capsules, as well as outer membrane proteins as antigens. Subsequently bioinformatics was employed to screen for proteins with epitope potential, conducted after the exposure of cell surface and highly conserved proteins. Moreover, translating findings from animal models to human clinical trials could be challenging due to variations in the expression levels of cytokines and differences in immunological checkpoints between humans and rodents [24]. Following these time-consuming and energy-intensive verification processes, very few successful candidates were identified [25,26].
Second, determining the applicable groups entails answering the question “what are the main target groups for vaccines?” Currently, only high-risk groups are targeted, such as patients in intensive care centers, patients with chronic diseases, those using ventilators, and individuals with cancer or undergoing surgical procedures. However, the protective effect during preoperative vaccination or at the onset of contracting diseases is limited. Therefore, defining high-risk groups in practice becomes challenging, potentially limiting the promotion of vaccines.
Last, the market for vaccines against MDR bacteria is currently not substantial. According to statistics from the United States (U.S.) Center for Disease Control and Prevention, approximately 2.8 million patients develop MDR bacterial infections every year [2]. Despite this large number, the incidence of developing such an infection is lower than that of other diseases; however, MDR bacterial infections still exhibit higher mortality rates. Moreover, the healthcare-related MDR bacterial infections have been proven to lead to an increase in the expensive healthcare expenses related to intensive care unit stays and prolonged hospital admissions [27]. Even so, in terms of economic benefits, biopharmaceutical companies are still encountering huge investment costs in R&D and considering short usage/profits lifespans, making this field less attractive when compared to the field of cancer treatment.
3. Developmental Process of Antibacterial Monoclonal Antibodies
MAbs are homogenous antibodies derived from a single B-cell clone, capable of detecting a single epitope in an antigen. In the past, while numerous antibiotics were available on the market, the cost of using mAbs as treatment options was excessively high. Therefore, in comparison with the fields of cancers and autoimmune diseases [29], the development of antibacterial mAbs has progressed relatively slower [30]. Currently, with the advancement of precision medicine and biotechnology, there is a growing demand for mAbs in anti-infective clinical applications. In comparison with antibiotics, the application of mAbs as treatment strategies for bacterial infections offers several advantages: (1) high specificity, precisely combating MDR bacteria; (2) high safety profile, without harming normal intestinal flora [31]; (3) the potential for combination with regular antibiotics (antibody–drug conjugates), thereby reducing the dose and presenting with selective pressure [32]; (4) affinity and safety of mAbs that can potentially be enhanced through genetic engineering, such as single-chain fragment variable (scFv) antibodies and fully human antibodies [33,34]; (5) long half-life, ensuring bioavailability for several weeks to months after administration, theoretically providing dosing, compliance, and adherence benefits [31]; (6) therapeutic advantages for immunocompromised patients and those for whom vaccination is inappropriate [35]; (7) production with minimal chemical usage compared to antibiotics, promoting environmental friendliness [36]; (8) easy degradation under various conditions, including temperature changes, pH shifts, or oxidation, thus preventing accumulation in the environment like antibiotics [37]; and (9) drug resistance is less likely to occur because they target specific virulence factors rather than essential survival proteins [38]. Furthermore, in several clinical trials, mAbs have been explored as potential adjuncts to antibiotic therapy. MAbs have the capacity to deliver antibiotics directly to the site of infection, mitigating the excess toxicity and collateral damage associated with antibiotic use. This approach not only minimizes antibiotic-related side effects but also allows for the reduction in antibiotic dosages. By reducing antibiotic dosages, the selection pressure exerted by antibiotics could be diminished, thereby decreasing the likelihood of MDR development. While these endeavors hold promise for improving treatment outcomes, the ultimate goal continues to be the reduction or replacement of antibiotic usage [32,39,40,41].
Thus far, mAbs can mainly be broadly categorized into mouse-derived, human–mouse chimera, humanized, and fully human mAbs, based on the type of development source [42] (Figure 1). The mouse-derived mAb is the hybridoma formed by the fusion of B lymphocytes of immunized mice and mouse myeloma cells. This represents the first generation of mAb preparation technology and is extensively used in antibody research. The human–mouse chimeric mAb is introduced into myeloma cells after genetic recombination between the variable region (Fv) gene on the mouse antibody and the constant region (Fc) of the human antibody, retaining approximately 30% of the murine antibody properties [34]. Humanized antibodies use the sequences of the complementarity determining regions (CDRs) in the mutant regions of murine mAbs to replace the corresponding positions in the variable regions of human antibodies, achieving approximately 90% humanization [43]. This type of antibody possesses the specificity of murine mAbs and retains affinity in humans. Fully human mAbs represent the most desirable option for mAb therapy. This category of mAb eliminates human heterogeneity across different species, thereby diminishing the risk of a human anti-chimeric antibody (HACA) response [33,34]. The preparation techniques for fully human mAbs mainly include the expression of the phage antibody library, ribosome display technology, and transgenic mouse technology [44]. In any case, mAbs are perceived as foreign antigens by the individual’s immune system, leading to the production of antibodies that can neutralize their effects or induce a pathological immune response. All chimeric mAbs inherently contain murine fragments, which inevitably produce HACA responses. While, humanized or fully human mAbs might elicit anti-drug antibody response, affecting pharmacokinetics (PKs) and mAb potency [31].
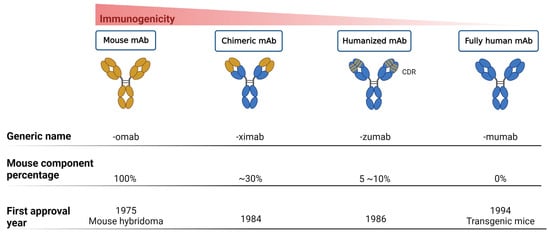
Figure 1. Progression of monoclonal antibody development. This figure illustrates the evolution of mAb technology, distinguishing between murine-derived antigens (indicated in yellow) and human-derived antigens (indicated in blue), to highlight the transition from animal-based to fully humanized antibody production for therapeutic use.
This figure delineates the evolution of mAbs, highlighting the reduction in immunogenicity from mouse to fully human mAbs. The upper bar graph illustrates the decreasing immunogenicity across four mAb generations: mouse, chimeric, humanized, and fully human mAbs. Below, three lines detail the generic name, mouse component percentage, and first approval year for each mAb type, showcasing the advancements in mAb development over time.
This entry is adapted from the peer-reviewed paper 10.3390/life14020246
This entry is offline, you can click here to edit this entry!
