Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Traumatic brain injury (TBI) affects approximately 250 in 100,000 individuals globally, contributing to 30–50% of trauma-related mortalities, with adolescents, young adults, and the elderly being the most affected groups. Mild TBI (mTBI) constitutes 80% of TBI cases, impacting around 42 million people annually, and can result in neuropsychiatric outcomes, impaired functionality, epilepsy, and neurodegenerative diseases.
- traumatic brain injury
- machine learning
- neuroimaging
- artificial intelligence
1. Introduction
Traumatic brain injury (TBI) affects approximately 250 in 100,000 individuals globally, contributing to 30–50% of trauma-related mortalities, with adolescents, young adults, and the elderly being the most affected groups [1][2]. Mild TBI (mTBI) constitutes 80% of TBI cases, impacting around 42 million people annually, and can result in neuropsychiatric outcomes, impaired functionality, epilepsy, and neurodegenerative diseases [3][4][5][6]. Despite the rising interest in accurate TBI and mTBI diagnosis through fluid and imaging biomarkers [5][7], limited research has focused on the sole use of computed tomography (CT) or magnetic resonance imaging (MRI) in TBI diagnosis. These modalities are widely accessible yet often demonstrate low sensitivity, especially for mTBI [8].
Recent advancements have witnessed a surge in TBI research utilizing machine learning, aiding in predicting mortality, long-term outcomes, intracranial pressure, and various other clinical parameters [9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55].
2. Machine Learning
Machine learning allows computers to learn from data, training algorithms to recognize patterns and make predictions on new, unseen data [56][57]. The typical workflow includes acquiring a dataset, addressing missing values, normalizing the data, choosing algorithms, selecting features, optimizing to avoid overfitting, and, finally, training, validating, testing, and choosing the optimal model [27].
There are three primary types of machine learning algorithms: supervised, unsupervised, and reinforcement learning (Figure 1). Supervised learning operates on labeled datasets, while unsupervised learning, which is particularly useful for discovering hidden structures in data, does not require labels. This makes unsupervised learning beneficial, as it sidesteps the often laborious and costly process of data annotation [57]. A specialized subset of machine learning, deep learning, leverages multi-layered artificial neural networks to understand intricate patterns in vast amounts of data.
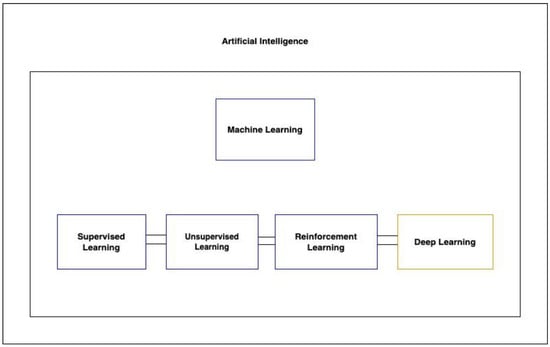
Figure 1. Showcases the hierarchy of artificial intelligence techniques, highlighting the relationships between AI, machine learning, its types, and deep learning.
In the context of TBI research, various algorithms have been employed, such as support vector machines (SVMs), artificial neural networks (ANNs), multilayer perceptrons (MPNs), and more. The choice of algorithm often depends on the nature of the data and the desired outcome. While selecting the right algorithm is crucial, choosing pertinent and predictive variables is equally, if not more, vital [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][40][41][42][43][44][46][47][48][49][50][51][52][53][54][55][58][59]. Typically, the performance of machine learning models in TBI research is assessed using metrics like sensitivity, specificity, accuracy, and area under the receiver-operating curve (AUROC).
3. Identifying mTBI Using Functional Brain Activity
The difficulty of detecting mTBI through imaging alone has given rise to a substantial body of literature exploring machine learning as a tool for diagnosis, particularly given mTBI’s association with dysregulated neural network functioning [60].
Vergara’s work stands out in this domain. In 2017, Vergara employed dynamic functional network connectivity (dFNC) using fMRI data to differentiate mTBI patients from controls. The ground truth for mTBI diagnosis was based on criteria from the American Congress of Rehabilitation Medicine, which included a Glasgow Coma Scale (GCS) between 13 and 15. They identified four separate dFNC states that a patient could occupy during the five-minute fMRI scan period. Using a linear SVM, participants were classified within each dFNC state into mTBI patients and healthy controls. They were able to achieve a 92% AUROC among 48 patients when classifying using the optimal dFNC state and observed increased dFNC in the cerebellum compared to sensorimotor networks [61]. Another study by the same team in 2016 used resting-state functional network connectivity (rsFNC) from fMRI and fractional anisotropy from diffusion MRI (dMRI), attaining accuracies of 84.1% and 75.5%, respectively, with an SVM model. Increased rsFNC was noted in specific brain regions. Static connectivity, however, as opposed to dFNC, is not able to take into account the dynamic properties of the brain [62]. Later, using SVMs trained with dNFC data on a cohort of 50 mTBI patients, they reported an AUROC of 73% for mTBI prediction [63].
Other researchers have also leveraged fMRI. Luo (2021) used SVMs on resting-state fMRI (rs-fMRI) data to distinguish mTBI from controls among 48 patients, achieving notable diagnostic metrics and focusing on parameters like amplitude of low-frequency fluctuation, degree centrality, and voxel-mirrored homotopic connectivity. By combining multiple such parameters, they were able to distinguish patients with mTBI from controls with an AUROC of 77.8% and accuracy of 81.11% [64]. Fan (2021) and Rangaprakash (2017, 2018) made similar attempts with rs-fMRI and SVM models, yielding accuracies of 0.74 and up to 0.84, respectively [63][65][66].
Vedaei et al. concentrated on chronic mTBI, emphasizing its diagnostic significance for guiding treatment. Incorporating rs-fMRI with various metrics in their machine learning approach, they reported AUCs ranging from 80.42 to 93.33% in a study with 100 participants [67].
4. Detecting Axonal Injury with Machine Learning
Several studies have utilized machine learning with diffusor tensor imaging (DTI) metrics to detect axonal injury, which appears to be a key determinant of clinical outcome. Hellyer (2013) and Fagerholm (2016) trained SVM models, focusing on variables like fractional anisotropy and radial diffusivity, to differentiate patients with microbleeds suggestive of axonal injury from controls, achieving sensitivities and specificities often above 0.90. Microbleeds are often considered a surrogate marker of traumatic axonal injury and are visible on gradient-echo and susceptibility-weighted imaging, but patients without microbleeds can still have axonal injury. Both studies extended their initial analyses on microbleed patients to the larger group of non-microbleed patients to show generalizability in predicting cognitive function in the larger group of patients when compared to neuropsychological testing [68][69]. Mitra (2016) utilized RF classifiers focusing on fractional anisotropy features to identify diffuse axonal injury in mTBI patients. Among 325 TBI (mean GCS 13.1) and control participants, they achieved a mean classification accuracy of around 68% and sensitivity of 80% [70].
Stone (2016) employed RF classifiers to segment and quantify white-matter hyperintensities, which have shown prognostic value in TBI, on fluid-attenuated inversion recovery (FLAIR) MRI sequences. They used an RF framework to segment images from 24 patients, achieving an accuracy of 0.68 [70][71]. Other studies by Bai (2020), Cai (2018), Minaee (2017), and Senyukova (2011) employed SVM models with DTI metrics, yielding accuracies ranging from 0.68 to 0.96 [72][73][74][75]. Abdelrahman (2022) combined SVM with principal component analysis (PCA) on DTI indices to classify 52 total TBI patients and controls, achieving an accuracy of 90.5% and AUC of 0.93 [76].
Machine learning’s potential extends to the clinical management of TBI patients with diffuse axonal injury (DAI). Mohamed (2022) employed a CNN to predict favorable or unfavorable outcomes using the Glasgow Outcome Scale (GOS) from MRIs of 38 patients who sustained moderate or severe TBI with MRI evidence of DAI, achieving a sensitivity of 0.997 and AUROC of 0.917 [77]. Bohyn (2022) applied the FDA-cleared machine learning software icobrain to calculate brain lesion volumes in 20 DAI patients, correlating white-matter volume changes with GOS [78]. Tjerkaski (2022) used an RF model with gradient-echo and susceptibility-weighted imaging to develop a novel MRI-based traumatic axonal injury (TAI) grading system to discern between favorable and unfavorable outcomes, achieving an AUC of 0.72 [39].
5. Predicting TBI with CT
CT remains a primary imaging modality for suspected TBI. The urgency of detecting lesions has spurred interest in machine learning to enhance and expedite diagnosis. Automated image analysis through machine learning can streamline the process, reducing the time and potential variability introduced by manual reviews.
Recent studies have showcased the potential of convolutional neural networks (CNNs) in this domain. Monteiro (2020) used CNNs to identify and quantify various types of intracranial hemorrhages, achieving sensitivity and specificity up to 0.8 and 0.9, respectively, for small lesions [79]. Keshavamurthy (2017) and Salehinejad (2021) trained SVM models and generalizable machine learning models, respectively, both demonstrating high accuracies and sensitivities in detecting characteristic TBI lesions on CT [80][81].
Midline shift (MLS) in CT due to space-occupying lesions (typically hematomas from intracranial hemorrhage in TBI patients) is a prognostic factor in TBI. Studies indicate that TBI patients with MLS less than 10 mm have notably better outcomes than those with more significant shifts [82]. Machine learning’s automated MLS measurement can minimize observer variability. Both Nag (2021) and Yan (2022) employed CNNs for MLS estimation, achieving commendable accuracies greater than 85% and consistency across different types of intracranial hemorrhages when compared to hand-drawn MLSs by clinicians across a range of MLS values from a 2 mm cutoff value to greater than 10 mm [83][84].
Predicting long-term outcomes post-TBI can guide critical clinical decisions. Pease (2022) combined a CNN model analyzing CT data with a clinical model to forecast 6-month outcomes in severe TBI. This fusion model outperformed the standard IMPACT model when tested on their internal dataset and even surpassed the predictions of three neurosurgeons [85].
6. Detecting and Quantifying Subdural Hematomas with Machine Learning
Machine learning aids in the segmentation of pathology from normal structures, and in the context of TBI, it offers potential in delineating subdural hematomas (SDHs). Accurate segmentation enables both detection and volumetric analysis. While manual volumetric evaluation can be labor-intensive and is often omitted in clinical practice, machine learning provides a time-efficient alternative. This is vital because SDH volume is crucial for prognosis and surgical intervention considerations and as a predictor of post-operative recurrence [86][87].
Recent endeavors have demonstrated the capabilities of machine learning in this area. Farzaneh (2020) utilized a random forest model to detect and classify the severity of acute SDHs, achieving a sensitivity of 0.99 and specificity of 0.92 [88]. Chen (2022) employed a CNN for volumetric assessment, with the results closely mirroring manual segmentation with an AUROC of 0.83 [89]. Another CNN model designed for comprehensive SDH evaluation, including thickness, volume, and midline shift, showcased a sensitivity of 91.4% and specificity of 96.4% [90].
Chronic SDHs (cSDHs) present unique challenges due to their varied densities and resemblance to brain parenchyma in certain phases [91]. Kellogg (2021) trained CNN models for both pre- and post-operative cSDHs, achieving a DICE score of 0.83 in predicting cSDH volumes [92]. Moreover, insights from a study by Kung et al. highlight the capabilities of machine learning in predicting post-operative recurrence of SDHs by analyzing specific pathological features [93].
This entry is adapted from the peer-reviewed paper 10.3390/traumacare4010004
References
- Fatuki, T.A.; Zvonarev, V.; Rodas, A.W. Prevention of Traumatic Brain Injury in the United States: Significance, New Findings, and Practical Applications. Cureus 2020, 12, e11225.
- Rodríguez-Triviño, C.Y.; Torres Castro, I.; Dueñas, Z. Hypochloremia in Patients with Severe Traumatic Brain Injury: A Possible Risk Factor for Increased Mortality. World Neurosurg. 2019, 124, e783–e788.
- McMahon, P.J.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T.; et al. Symptomatology and Functional Outcome in Mild Traumatic Brain Injury: Results from the Prospective TRACK-TBI Study. J. Neurotrauma 2014, 31, 26–33.
- Keret, A.; Bennett-Back, O.; Rosenthal, G.; Gilboa, T.; Shweiki, M.; Shoshan, Y.; Benifla, M. Posttraumatic epilepsy: Long-term follow-up of children with mild traumatic brain injury. J. Neurosurg. Pediatr. PED 2017, 20, 64–70.
- Pierre, K.; Dyson, K.; Dagra, A.; Williams, E.; Porche, K.; Lucke-Wold, B. Chronic Traumatic Encephalopathy: Update on Current Clinical Diagnosis and Management. Biomedicines 2021, 9, 415.
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80.
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert. Rev. Mol. Diagn. 2018, 18, 165–180.
- Shenton, M.E.; Hamoda, H.M.; Schneiderman, J.S.; Bouix, S.; Pasternak, O.; Rathi, Y.; Vu, M.A.; Purohit, M.P.; Helmer, K.; Koerte, I.; et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 137–192.
- Wu, X.; Sun, Y.; Xu, X.; Steyerberg, E.W.; Helmrich, I.R.A.R.; Lecky, F.; Guo, J.; Li, X.; Feng, J.; Mao, Q.; et al. Mortality Prediction in Severe Traumatic Brain Injury Using Traditional and Machine Learning Algorithms. J. Neurotrauma 2023, 40, 1366–1375.
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients on mechanical ventilation post traumatic brain injury: Machine learning approach. BMC Med. Inform. Decis. Mak. 2020, 20, 336.
- Tunthanathip, T.; Oearsakul, T. Application of machine learning to predict the outcome of pediatric traumatic brain injury. Chin. J. Traumatol. 2021, 24, 350–355.
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients with post traumatic brain injury using National Trauma Registry and Machine Learning Approach. Scand. J. Trauma. Resusc. Emerg. Med. 2020, 28, 44.
- Hsu, S.-D.; Chao, E.; Chen, S.-J.; Hueng, D.-Y.; Lan, H.-Y.; Chiang, H.-H. Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury. J. Pers. Med. 2021, 11, 1144.
- Folweiler, K.A.; Sandsmark, D.K.; Diaz-Arrastia, R.; Cohen, A.S.; Masino, A.J. Unsupervised Machine Learning Reveals Novel Traumatic Brain Injury Patient Phenotypes with Distinct Acute Injury Profiles and Long-Term Outcomes. J. Neurotrauma 2020, 37, 1431–1444.
- Hale, A.T.; Stonko, D.P.; Brown, A.; Lim, J.; Voce, D.J.; Gannon, S.R.; Le, T.M.; Shannon, C.N. Machine-learning analysis outperforms conventional statistical models and CT classification systems in predicting 6-month outcomes in pediatric patients sustaining traumatic brain injury. Neurosurg. Focus FOC 2018, 45, E2.
- Hernandes Rocha, T.A.; Elahi, C.; Cristina da Silva, N.; Sakita, F.M.; Fuller, A.; Mmbaga, B.T.; Green, E.P.; Haglund, M.M.; Staton, C.A.; Nickenig Vissoci, J.R. A traumatic brain injury prognostic model to support in-hospital triage in a low-income country: A machine learning–based approach. J. Neurosurg. JNS 2020, 132, 1961–1969.
- Vishwanath, M.; Jafarlou, S.; Shin, I.; Lim, M.M.; Dutt, N.; Rahmani, A.M.; Cao, H. Investigation of Machine Learning Approaches for Traumatic Brain Injury Classification via EEG Assessment in Mice. Sensors 2020, 20, 2027.
- Farzaneh, N.; Williamson, C.A.; Gryak, J.; Najarian, K. A hierarchical expert-guided machine learning framework for clinical decision support systems: An application to traumatic brain injury prognostication. NPJ Digit. Med. 2021, 4, 78.
- Raj, R.; Wennervirta, J.M.; Tjerkaski, J.; Luoto, T.M.; Posti, J.P.; Nelson, D.W.; Takala, R.; Bendel, S.; Thelin, E.P.; Luostarinen, T.; et al. Dynamic prediction of mortality after traumatic brain injury using a machine learning algorithm. NPJ Digit. Med. 2022, 5, 96.
- Warman, P.I.; Seas, A.; Satyadev, N.; Adil, S.M.; Kolls, B.J.; Haglund, M.M.; Dunn, T.W.; Fuller, A.T. Machine Learning for Predicting In-Hospital Mortality After Traumatic Brain Injury in Both High-Income and Low- and Middle-Income Countries. Neurosurgery 2022, 90, 605–612.
- Bruschetta, R.; Tartarisco, G.; Lucca, L.F.; Leto, E.; Ursino, M.; Tonin, P.; Pioggia, G.; Cerasa, A. Predicting Outcome of Traumatic Brain Injury: Is Machine Learning the Best Way? Biomedicines 2022, 10, 686.
- Satyadev, N.; Warman, P.I.; Seas, A.; Kolls, B.J.; Haglund, M.M.; Fuller, A.T.; Dunn, T.W. Machine Learning for Predicting Discharge Disposition After Traumatic Brain Injury. Neurosurgery 2022, 90, 768–774.
- Lang, E.; Neuschwander, A.; Favé, G.; Abback, P.-S.; Esnault, P.; Geeraerts, T.; Harrois, A.; Hanouz, J.-L.; Kipnis, E.; Leone, M.; et al. Clinical decision support for severe trauma patients: Machine learning based definition of a bundle of care for hemorrhagic shock and traumatic brain injury. J. Trauma Acute Care Surg. 2022, 92, 135–143.
- Mohd Noor, N.S.E.; Ibrahim, H. Predicting Outcomes in Patients with Traumatic Brain Injury Using Machine Learning Models. In Proceedings of the Intelligent Manufacturing and Mechatronics, Melaka, Malaysia, 8 July 2019; Springer: Singapore, 2020; pp. 12–20.
- Radabaugh, H.L.; Bonnell, J.; Dietrich, W.D.; Bramlett, H.M.; Schwartz, O.; Sarkar, D. Development and Evaluation of Machine Learning Models for Recovery Prediction after Treatment for Traumatic Brain Injury. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2416–2420.
- Lee, S.H.; Lee, C.H.; Hwang, S.H.; Kang, D.H. A Machine Learning–Based Prognostic Model for the Prediction of Early Death After Traumatic Brain Injury: Comparison with the Corticosteroid Randomization After Significant Head Injury (CRASH) Model. World Neurosurg. 2022, 166, e125–e134.
- Guimarães, K.A.A.; de Amorim, R.L.O.; Costa, M.G.F.; Costa Filho, C.F.F. Predicting early traumatic brain injury mortality with 1D convolutional neural networks and conventional machine learning techniques. Inform. Med. Unlocked 2022, 31, 100984.
- Amorim, R.L.; Oliveira, L.M.; Malbouisson, L.M.; Nagumo, M.M.; Simoes, M.; Miranda, L.; Bor-Seng-Shu, E.; Beer-Furlan, A.; De Andrade, A.F.; Rubiano, A.M.; et al. Prediction of Early TBI Mortality Using a Machine Learning Approach in a LMIC Population. Front. Neurol. 2019, 10, 1366.
- Signorini, D.F.; Andrews, P.J.; Jones, P.A.; Wardlaw, J.M.; Miller, J.D. Predicting survival using simple clinical variables: A case study in traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 1999, 66, 20–25.
- Pang, B.C.; Kuralmani, V.; Joshi, R.; Hongli, Y.; Lee, K.K.; Ang, B.T.; Li, J.; Leong, T.Y.; Ng, I. Hybrid Outcome Prediction Model for Severe Traumatic Brain Injury. J. Neurotrauma 2007, 24, 136–146.
- Chong, S.L.; Liu, N.; Barbier, S.; Ong, M.E. Predictive modeling in pediatric traumatic brain injury using machine learning. BMC Med. Res. Methodol. 2015, 15, 22.
- Adil, S.M.; Elahi, C.; Gramer, R.; Spears, C.A.; Fuller, A.T.; Haglund, M.M.; Dunn, T.W. Predicting the Individual Treatment Effect of Neurosurgery for Patients with Traumatic Brain Injury in the Low-Resource Setting: A Machine Learning Approach in Uganda. J. Neurotrauma 2020, 38, 928–939.
- Daley, M.; Cameron, S.; Ganesan, S.L.; Patel, M.A.; Stewart, T.C.; Miller, M.R.; Alharfi, I.; Fraser, D.D. Pediatric severe traumatic brain injury mortality prediction determined with machine learning-based modeling. Injury 2022, 53, 992–998.
- Nourelahi, M.; Dadboud, F.; Khalili, H.; Niakan, A.; Parsaei, H. A machine learning model for predicting favorable outcome in severe traumatic brain injury patients after 6 months. Acute Crit. Care 2022, 37, 45–52.
- Matsuo, K.; Aihara, H.; Nakai, T.; Morishita, A.; Tohma, Y.; Kohmura, E. Machine Learning to Predict In-Hospital Morbidity and Mortality after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 202–210.
- Feng, J.; Wang, Y.; Peng, J.; Sun, M.; Zeng, J.; Jiang, H. Comparison between logistic regression and machine learning algorithms on survival prediction of traumatic brain injuries. J. Crit. Care 2019, 54, 110–116.
- Noor, N.S.E.M.; Ibrahim, H. Machine Learning Algorithms and Quantitative Electroencephalography Predictors for Outcome Prediction in Traumatic Brain Injury: A Systematic Review. IEEE Access 2020, 8, 102075–102092.
- Palacios, E.M.; Owen, J.P.; Yuh, E.L.; Wang, M.B.; Vassar, M.J.; Ferguson, A.R.; Diaz-Arrastia, R.; Giacino, J.T.; Okonkwo, D.O.; Robertson, C.S.; et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci. Adv. 2020, 6, eaaz6892.
- Tjerkaski, J.; Nyström, H.; Raj, R.; Lindblad, C.; Bellander, B.-M.; Nelson, D.W.; Thelin, E.P. Extended Analysis of Axonal Injuries Detected Using Magnetic Resonance Imaging in Critically Ill Traumatic Brain Injury Patients. J. Neurotrauma 2022, 39, 58–66.
- Chen, W.; Cockrell, C.; Ward, K.R.; Najarian, K. Intracranial pressure level prediction in traumatic brain injury by extracting features from multiple sources and using machine learning methods. In Proceedings of the 2010 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Hong Kong, China, 18–21 December 2010; pp. 510–515.
- Ye, G.; Balasubramanian, V.; Li, J.K.J.; Kaya, M. Machine Learning-Based Continuous Intracranial Pressure Prediction for Traumatic Injury Patients. IEEE J. Transl. Eng. Health Med. 2022, 10, 4901008.
- Chen, W.; Cockrell, C.H.; Ward, K.; Najarian, K. Predictability of intracranial pressure level in traumatic brain injury: Features extraction, statistical analysis and machine learning-based evaluation. Int. J. Data Min. Bioinform. 2013, 8, 480–494.
- Tunthanathip, T.; Duangsuwan, J.; Wattanakitrungroj, N.; Tongman, S.; Phuenpathom, N. Comparison of intracranial injury predictability between machine learning algorithms and the nomogram in pediatric traumatic brain injury. Neurosurg. Focus 2021, 51, E7.
- Molaei, S.; Korley, F.K.; Soroushmehr, S.M.R.; Falk, H.; Sair, H.; Ward, K.; Najarían, K. A machine learning based approach for identifying traumatic brain injury patients for whom a head CT scan can be avoided. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2258–2261.
- Li, Y.-C.; Liu, L.; Chiu, W.-T.; Jian, W.-S. Neural network modeling for surgical decisions on traumatic brain injury patients. Int. J. Med. Inform. 2000, 57, 1–9.
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Using trauma registry data to predict prolonged mechanical ventilation in patients with traumatic brain injury: Machine learning approach. PLoS ONE 2020, 15, e0235231.
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Al-Thani, H.; El-Menyar, A. Machine Learning Model to Predict Ventilator Associated Pneumonia in patients with Traumatic Brain Injury: The C.5 Decision Tree Approach. Brain Inj. 2021, 35, 1095–1102.
- Marincowitz, C.; Paton, L.; Lecky, F.; Tiffin, P. Predicting need for hospital admission in patients with traumatic brain injury or skull fractures identified on CT imaging: A machine learning approach. Emerg. Med. J. 2022, 39, 394–401.
- Abujaber, A.; Fadlalla, A.; Nashwan, A.; El-Menyar, A.; Al-Thani, H. Predicting prolonged length of stay in patients with traumatic brain injury: A machine learning approach. Intell.-Based Med. 2022, 6, 100052.
- Prichep, L.S.; Jacquin, A.; Filipenko, J.; Dastidar, S.G.; Zabele, S.; Vodencarević, A.; Rothman, N.S. Classification of traumatic brain injury severity using informed data reduction in a series of binary classifier algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 806–822.
- Yadav, K.; Sarioglu, E.; Choi, H.A.; Cartwright Iv, W.B.; Hinds, P.S.; Chamberlain, J.M. Automated Outcome Classification of Computed Tomography Imaging Reports for Pediatric Traumatic Brain Injury. Acad. Emerg. Med. 2016, 23, 171–178.
- Ellethy, H.; Chandra, S.S.; Nasrallah, F.A. The detection of mild traumatic brain injury in paediatrics using artificial neural networks. Comput. Biol. Med. 2021, 135, 104614.
- Dhillon, N.S.; Sutandi, A.; Vishwanath, M.; Lim, M.M.; Cao, H.; Si, D. A Raspberry Pi-Based Traumatic Brain Injury Detection System for Single-Channel Electroencephalogram. Sensors 2021, 21, 2779.
- Peacock, W.F.; Van Meter, T.E.; Mirshahi, N.; Ferber, K.; Gerwien, R.; Rao, V.; Sair, H.I.; Diaz-Arrastia, R.; Korley, F.K. Derivation of a Three Biomarker Panel to Improve Diagnosis in Patients with Mild Traumatic Brain Injury. Front. Neurol. 2017, 8, 641.
- Rau, C.S.; Kuo, P.J.; Chien, P.C.; Huang, C.Y.; Hsieh, H.Y.; Hsieh, C.H. Mortality prediction in patients with isolated moderate and severe traumatic brain injury using machine learning models. PLoS ONE 2018, 13, e0207192.
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine Learning for Medical Imaging. Radiographics 2017, 37, 505–515.
- Wagner, M.W.; Namdar, K.; Biswas, A.; Monah, S.; Khalvati, F.; Ertl-Wagner, B.B. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology 2021, 63, 1957–1967.
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, J.D.F.; et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med. 2008, 5, e165.
- Perel, P.; Arango, M.; Clayton, T.; Edwards, P.; Komolafe, E.; Poccock, S.; Roberts, I.; Shakur, H.; Steyerberg, E.; Yutthakasemsunt, S. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429.
- Safar, K.; Zhang, J.; Emami, Z.; Gharehgazlou, A.; Ibrahim, G.; Dunkley, B.T. Mild traumatic brain injury is associated with dysregulated neural network functioning in children and adolescents. Brain Commun. 2021, 3, fcab044.
- Vergara, V.M.; Mayer, A.R.; Kiehl, K.A.; Calhoun, V.D. Dynamic functional network connectivity discriminates mild traumatic brain injury through machine learning. NeuroImage Clin. 2018, 19, 30–37.
- Vergara, V.M.; Mayer, A.R.; Damaraju, E.; Kiehl, K.A.; Calhoun, V. Detection of Mild Traumatic Brain Injury by Machine Learning Classification Using Resting State Functional Network Connectivity and Fractional Anisotropy. J. Neurotrauma 2017, 34, 1045–1053.
- Rangaprakash, D.; Dretsch, M.N.; Venkataraman, A.; Katz, J.S.; Denney, T.S., Jr.; Deshpande, G. Identifying disease foci from static and dynamic effective connectivity networks: Illustration in soldiers with trauma. Hum. Brain Mapp. 2018, 39, 264–287.
- Luo, X.; Lin, D.; Xia, S.; Wang, D.; Weng, X.; Huang, W.; Ye, H. Machine Learning Classification of Mild Traumatic Brain Injury Using Whole-Brain Functional Activity: A Radiomics Analysis. Dis. Markers 2021, 2021, 3015238.
- Fan, L.; Xu, H.; Su, J.; Qin, J.; Gao, K.; Ou, M.; Peng, S.; Shen, H.; Li, N. Discriminating mild traumatic brain injury using sparse dictionary learning of functional network dynamics. Brain Behav. 2021, 11, e2414.
- Rangaprakash, D.; Deshpande, G.; Daniel, T.A.; Goodman, A.M.; Robinson, J.L.; Salibi, N.; Katz, J.S.; Denney, T.S., Jr.; Dretsch, M.N. Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum. Brain Mapp. 2017, 38, 2843–2864.
- Vedaei, F.; Mashhadi, N.; Zabrecky, G.; Monti, D.; Navarreto, E.; Hriso, C.; Wintering, N.; Newberg, A.B.; Mohamed, F.B. Identification of chronic mild traumatic brain injury using resting state functional MRI and machine learning techniques. Front. Neurosci. 2022, 16, 1099560.
- Hellyer, P.J.; Leech, R.; Ham, T.E.; Bonnelle, V.; Sharp, D.J. Individual prediction of white matter injury following traumatic brain injury. Ann. Neurol. 2013, 73, 489–499.
- Fagerholm, E.D.; Hellyer, P.J.; Scott, G.; Leech, R.; Sharp, D.J. Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain 2015, 138 Pt 6, 1696–1709.
- Mitra, J.; Shen, K.-k.; Ghose, S.; Bourgeat, P.; Fripp, J.; Salvado, O.; Pannek, K.; Taylor, D.J.; Mathias, J.L.; Rose, S. Statistical machine learning to identify traumatic brain injury (TBI) from structural disconnections of white matter networks. NeuroImage 2016, 129, 247–259.
- Stone, J.R.; Wilde, E.A.; Taylor, B.A.; Tate, D.F.; Levin, H.; Bigler, E.D.; Scheibel, R.S.; Newsome, M.R.; Mayer, A.R.; Abildskov, T.; et al. Supervised learning technique for the automated identification of white matter hyperintensities in traumatic brain injury. Brain Inj. 2016, 30, 1458–1468.
- Bai, L.; Bai, G.; Wang, S.; Yang, X.; Gan, S.; Jia, X.; Yin, B.; Yan, Z. Strategic white matter injury associated with long-term information processing speed deficits in mild traumatic brain injury. Hum. Brain Mapp. 2020, 41, 4431–4441.
- Cai, Y.; Wu, S.; Zhao, W.; Li, Z.; Wu, Z.; Ji, S. Concussion classification via deep learning using whole-brain white matter fiber strains. PLoS ONE 2018, 13, e0197992.
- Minaee, S.; Wang, Y.; Chung, S.; Wang, X.H.; Fieremans, E.; Flanagan, S.; Rath, J.F.; Lui, Y.W. A Machine Learning Approach For Identifying Patients with Mild Traumatic Brain Injury Using Diffusion MRI Modeling. arXiv 2017, arXiv:1708.09000.
- Senyukova, O.; Galanine, V.; Krylov, A.; Petraikin, A.; Akhadov, T.; Sidorin, S. Diffuse Axonal Injury Lesion Segmentation Using Contouring Algorithm. In Proceedings of the 21st International Conference on Computer Graphics and Vision, GraphiCon’2011-Conference Proceedings, Moscow, Russia, 26–30 September 2011.
- Abdelrahman, H.A.F.; Ubukata, S.; Ueda, K.; Fujimoto, G.; Oishi, N.; Aso, T.; Murai, T. Combining Multiple Indices of Diffusion Tensor Imaging Can Better Differentiate Patients with Traumatic Brain Injury from Healthy Subjects. Neuropsychiatr. Dis. Treat. 2022, 18, 1801–1814.
- Mohamed, M.; Alamri, A.; Mohamed, M.; Khalid, N.; O’Halloran, P.; Staartjes, V.; Uff, C. Prognosticating outcome using magnetic resonance imaging in patients with moderate to severe traumatic brain injury: A machine learning approach. Brain Inj. 2022, 36, 353–358.
- Bohyn, C.; Vyvere, T.V.; Keyzer, F.; Sima, D.M.; Demaerel, P. Morphometric evaluation of traumatic axonal injury and the correlation with post-traumatic cerebral atrophy and functional outcome. Neuroradiol. J. 2022, 35, 468–476.
- Monteiro, M.; Newcombe, V.F.J.; Mathieu, F.; Adatia, K.; Kamnitsas, K.; Ferrante, E.; Das, T.; Whitehouse, D.; Rueckert, D.; Menon, D.K.; et al. Multiclass semantic segmentation and quantification of traumatic brain injury lesions on head CT using deep learning: An algorithm development and multicentre validation study. Lancet Digit. Health 2020, 2, e314–e322.
- Keshavamurthy, K.N.; Leary, O.P.; Merck, L.H.; Kimia, B.B.; Collins, S.; Wright, D.W.; Allen, J.W.; Brock, J.F.; Merck, D. Machine learning algorithm for automatic detection of CT-identifiable hyperdense lesions associated with traumatic brain injury. In Proceedings of the Medical Imaging 2017: Computer-Aided Diagnosis, Orlando, FL, USA, 13–16 February 2017.
- Salehinejad, H.; Kitamura, J.; Ditkofsky, N.; Lin, A.; Bharatha, A.; Suthiphosuwan, S.; Lin, H.M.; Wilson, J.R.; Mamdani, M.; Colak, E. A real-world demonstration of machine learning generalizability in the detection of intracranial hemorrhage on head computerized tomography. Sci. Rep. 2021, 11, 17051.
- Puffer, R.C.; Yue, J.K.; Mesley, M.; Billigen, J.B.; Sharpless, J.; Fetzick, A.L.; Puccio, A.; Diaz-Arrastia, R.; Okonkwo, D.O. Long-term outcome in traumatic brain injury patients with midline shift: A secondary analysis of the Phase 3 COBRIT clinical trial. J. Neurosurg. 2018, 131, 596–603.
- Nag, M.K.; Gupta, A.; Hariharasudhan, A.S.; Sadhu, A.K.; Das, A.; Ghosh, N. Quantitative analysis of brain herniation from non-contrast CT images using deep learning. J. Neurosci. Methods 2021, 349, 109033.
- Yan, J.L.; Chen, Y.L.; Chen, M.Y.; Chen, B.A.; Chang, J.X.; Kao, C.C.; Hsieh, M.C.; Peng, Y.T.; Huang, K.C.; Chen, P.Y. A Robust, Fully Automatic Detection Method and Calculation Technique of Midline Shift in Intracranial Hemorrhage and Its Clinical Application. Diagnostics 2022, 12, 693.
- Pease, M.; Arefan, D.; Barber, J.; Yuh, E.; Puccio, A.; Hochberger, K.; Nwachuku, E.; Roy, S.; Casillo, S.; Temkin, N.; et al. Outcome Prediction in Patients with Severe Traumatic Brain Injury Using Deep Learning from Head CT Scans. Radiology 2022, 304, 385–394.
- Stanišić, M.; Hald, J.; Rasmussen, I.A.; Pripp, A.H.; Ivanović, J.; Kolstad, F.; Sundseth, J.; Züchner, M.; Lindegaard, K.F. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: A prospective study of 107 operated patients. Acta Neurochir. 2013, 155, 323–333; discussion 333.
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J.E. Surgical management of acute subdural hematomas. Neurosurgery 2006, 58, S16–S24; discussion Si-iv.
- Farzaneh, N.; Williamson, C.A.; Jiang, C.; Srinivasan, A.; Bapuraj, J.R.; Gryak, J.; Najarian, K.; Soroushmehr, S.M.R. Automated Segmentation and Severity Analysis of Subdural Hematoma for Patients with Traumatic Brain Injuries. Diagnostics 2020, 10, 773.
- Chen, D.; Bian, L.; He, H.Y.; Li, Y.D.; Ma, C.; Mao, L.G. Evaluation of Traumatic Subdural Hematoma Volume by Using Image Segmentation Assessment Based on Deep Learning. Comput. Math. Methods Med. 2022, 2022, 3830245.
- Colasurdo, M.; Leibushor, N.; Robledo, A.; Vasandani, V.; Luna, Z.A.; Rao, A.S.; Garcia, R.; Srinivasan, V.M.; Sheth, S.A.; Avni, N.; et al. Automated detection and analysis of subdural hematomas using a machine learning algorithm. J. Neurosurg. 2023, 138, 1077–1084.
- Khalili, H.; Rismani, M.; Nematollahi, M.A.; Masoudi, M.S.; Asadollahi, A.; Taheri, R.; Pourmontaseri, H.; Valibeygi, A.; Roshanzamir, M.; Alizadehsani, R.; et al. Prognosis prediction in traumatic brain injury patients using machine learning algorithms. Sci. Rep. 2023, 13, 960.
- Kellogg, R.T.; Vargas, J.; Barros, G.; Sen, R.; Bass, D.; Mason, J.R.; Levitt, M. Segmentation of Chronic Subdural Hematomas Using 3D Convolutional Neural Networks. World Neurosurg. 2021, 148, e58–e65.
- Kung, W.M.; Lin, M.S. CT-Based Quantitative Analysis for Pathological Features Associated With Postoperative Recurrence and Potential Application Upon Artificial Intelligence: A Narrative Review With a Focus on Chronic Subdural Hematomas. Mol. Imaging 2020, 19, 1536012120914773.
This entry is offline, you can click here to edit this entry!
