Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Exosomes, ranging from 30 to 150 nanometers in diameter, emerge as crucial biological nano-scale lipid bilayer vesicles. These vesicles are secreted by various cell types, including dendritic cells, macrophages, B cells, T cells, mesenchymal stem cells, endothelial cells, epithelial cells, and several cancer cells.
- exosomes
- circulating exosomes
- lung cancer
- NSCLC
- diagnosis
- biomarkers
1. Introduction
Cancer, as stated by the World Health Organization (WHO), stands as a leading cause of mortality worldwide [1]. Specifically, lung cancer is among the most reported cancer-related deaths globally [2][3], constituting 11.6% of new cancer diagnoses and accounting for 19.8% of cancer-related fatalities [4][5].
Regrettably, despite a decline in lung cancer mortality rates, a significant proportion of patients continue to receive diagnoses in advanced or metastatic stages, resulting in poor prognoses [6]. Advanced stages pose greater therapeutic challenges and exhibit a heightened propensity for developing resistance to treatment. Various treatment modalities have been employed in lung cancer care, each with its own advantages and drawbacks. Surgery remains a viable option for early-stage cases [7]. Chemotherapy, radiotherapy, and immunotherapy, either individually or in combination, are typical approaches for both early and advanced stages [8][9][10]. The differential factor lies in the responsiveness of lung cancer cells to these treatments, with a higher sensitivity observed during the early stages.
Hence, the study of tumorigenesis and therapeutic strategies against cancers, particularly lung cancer, is underscored. Recent research has yielded fresh insights into lung cancer biology, yielding notable progress in the realm of targeted therapies focused on novel biomarkers. These include molecular therapies targeting the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), proto-oncogene B-Raf (BRAF), and immunotherapies employing checkpoint inhibitors against the programmed cell death 1 (PD-1) and programmed death-ligand 1 (PD-L1) pathways [8][11][12]. Nonetheless, lung cancer still lags behind with one of the lowest 5-year survival rates, standing at a mere 18%, among all cancer types [9].
Extracellular vesicles (EVs), which are a collective term covering exosomes, microvesicles (MVs), microparticles, ectosomes, oncosomes, and apoptotic bodies, were first discovered by Pan and Johnson during the maturation of sheep reticulocytes in 1983 [13]. EVs are defined as heterogeneous entities of phospholipid bilayer membrane-bound vesicles without any means of replication [14]. They were initially thought to be “cellular dust” or served as a mechanism by which cells actively dispose of their own waste [15]. In 1996, exosomes were found to play a role in intercellular communication [16]. Due to the heterogeneity between and within exosome types and the overlap in characteristics between exosomes and other EVs, it is difficult to define exosomes in a way that distinguishes them from other EVs. Over the past few years, it has been discovered that exosomes, which contain proteins and miRNAs with biological effects, have the ability to specifically transport and deliver these bioactive cargoes to tumor cells [15].
Exosomes, ranging from 30 to 150 nanometers in diameter, emerge as crucial biological nano-scale lipid bilayer vesicles [17][18]. These vesicles are secreted by various cell types, including dendritic cells, macrophages, B cells, T cells, mesenchymal stem cells, endothelial cells, epithelial cells, and several cancer cells. They float within a sucrose density gradient solution at a density of 1.13–1.19 g mL−1 and carry an array of biomolecules, such as proteins (either membrane-bound or encapsulated within the vesicle), RNA (comprising coding mRNA or diverse non-coding RNAs), DNA (both double-stranded and single-stranded), as well as glycans [16][19][20]. They have been reported in all biological fluids, and the composition of the complex cargo of exosomes is readily accessible via the sampling of biological fluids (liquid biopsies) [21][22]. Cumulative evidence has revealed that exosomes play an irreplaceable role in prognostic, diagnostic, and even therapeutic aspects [21][23]. Nanotechnology has been employed for drug delivery for increasing the bioavailability of therapeutic agents. Unfortunately, drug nanoformulations often lead to toxicity and are usually rapidly cleared through the mononuclear phagocytic system (MPS) [24][25].
EVs can be categorized, based on their size, into microvesicles (MVs; 100–1000 nm) and exosomes (EXOs; 30–150 nm). They play pivotal roles in immune responses, angiogenesis, neuronal regeneration, anti-inflammation, coagulation, and in the intracellular degradation pathways [14][26].
Exosomes’ unique properties make them promising tools for therapeutically targeting diseases, including neurodegenerative conditions and various cancers [27]. They have shown promise as non-invasive biomarkers for chronic pain mechanisms [28] and as potential carriers for biomarkers and drugs in conditions such as human immunodeficiency virus (HIV) [29]. They can cross biological barriers, including the blood–brain barrier, and have neuroprotective and tissue repair effects [30][31]. It has been demonstrated that exosomes have potential applications in a wide range of fields, from oncology (lung, liver, pancreatic, colorectal, gastric, kidney, bladder, prostate, breast, ovarian, cervical, head and neck, thyroid, glioma, melanoma, and hematological malignancies) to neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, Huntington’s diseases, and amyotrophic lateral sclerosis (ALS), mental health conditions, cardiovascular diseases, diabetes, and inflammatory/autoimmune disorders [27].
2. The Biogenesis of Exosomes
Exosomes are small vesicles generated from late endosomes, which are formed via the inward budding of the multivesicular body (MVB) membrane. They are different from other vesicles called MVs, which are larger and generated via outward budding from the plasma membrane [32]. Exosomes have an approximate size range from 30 nm to 150 nm [17][18] and contain proteins, mRNAs, microRNAs, and lipids. They are derived from various cellular sources and have biomarkers such as TSG101, CHMP2A, RAB11B, CD63, and CD81 [33]. Exosomes play a crucial role in cell-to-cell communication and are involved in various biological processes and diseases [34]. They can escape clearance by the immune system and have a longer circulation time compared to other vesicles [32]. The formation of exosomes involves the invagination of late endosomal membranes, resulting in the formation of intraluminal vesicles (ILVs) within MVBs [35]. ILVs are eventually secreted as exosomes through fusion of MVBs with the plasma membrane [36]. The content of exosomes depends on the cell type they originate from and can include proteins and components from various cellular compartments [33].
Exosomes have a distinct shape, appearing biconcave or cup-like when dried and spheroid when observed under a microscope [32]. Exosomes can form early-sorting endosomes (ESEs) de novo or merge with existing ESEs. The trans-Golgi network and endoplasmic reticulum contribute to ESE formation. ESEs mature into late-sorting endosomes (LSEs) and eventually generate MVBs, also known as multivesicular endosomes [20]. During maturation, the content of endosomes becomes acidic, and markers such as Rab5 are replaced by Rab7, Rab9, and the mannose-6-phosphate receptor to indicate late endosomes [35]. The influence of specific markers or cargo on these pathways is still unclear [37].
Several mechanisms contribute to exosome generation. The endosomal sorting complexes required for transport (ESCRT-0, -I, -II, and -III) and the ATPase Vps4 complex are important in this process [38]. SNARE proteins and RAB GTPases are also involved in exosome secretion [39][40]. Even when key subunits of ESCRTs are depleted, ESCRT-independent ILV biogenesis can still occur [41]. Tetraspanins and lipids play significant roles in exosome generation and release [42]. Identifying molecules that regulate exosome biogenesis could lead to therapeutic targets for controlling exosome secretion and modulating related signaling pathways in conditions such as metastasis and inflammation [43][44]. Cholesterol treatment in hepatoma cells reduces MVBs and increases exosome secretion with the ability to induce specific immune responses. Statins, known for lowering cholesterol, have been shown to reduce exosome release, suggesting their potential as therapeutic agents to control exosome production in specific cells [42].
Exosome biogenesis and release are influenced by various molecules, including components of the ESCRT machinery, Rab GTPases, tetraspanins, and the adaptor protein syntenin. The ESCRT mechanism comprises four separate protein complexes (ESCRT-0 through III) that work together to facilitate the formation of MVBs, vesicle budding, and sorting of protein cargo. Ubiquitinated proteins are recognized and sequestered to specific domains of the endosomal membrane by the ubiquitin-binding subunits of ESCRT-0, initiating the process. ESCRT-I and ESCRT-II complexes interact with ESCRT-III to promote budding. The ESCRT-III complex then separates from the MVB membrane, aided by the Vps4 sorting protein, after cleaving the buds to form ILVs [32][45].
In a study using HeLa cells, an RNAi screen targeting twenty-three ESCRT and ESCRT-associated proteins identified seven proteins that impacted exosome secretion. The depletion of ESCRT-0 proteins (Hrs and TSG101) and the ESCRT-I protein STAM1 reduced exosome secretion, while the knockdown of ESCRT-III and its associated proteins (CHMP4C, VPS4B, VTA1, and ALIX) increased exosome secretion. Further analysis verified the role of these proteins, with Hrs, TSG101, and STAM1 depletion decreasing exosome secretion and VPS4B knockdown increasing it. ALIX depletion appeared to affect the protein composition of exosomes rather than secretion, suggesting it may impact cargo loading and the types of MVBs destined for secretion [46][47].
The interaction between syntenin and ALIX is important for sorting syndecans, membrane proteins with heparan sulfate chains, into exosomes. Syndecan sorting and ILV formation are facilitated by syntenin, which binds to both syndecans and ALIX [47][48]. Heparanases trim the heparan sulfate chains, promoting the formation of syndecan clusters that enhance binding to syntenin. Heparanase also stimulates the sorting of CD63, indicating a potential relationship between the sorting of these two molecules [47][49]. The syndecan–syntenin–ALIX mechanism is estimated to control around 50% of secreted vesicles in MCF-7 cells, suggesting the involvement of different sorting mechanisms in exosomal molecule sorting [47][50]. These findings suggest that ESCRT function plays a role in exosomal biogenesis.
Cellular metabolic status, including ceramide metabolism, ER stress, autophagy, and intracellular calcium levels, can impact exosome production. Adiponectin, a protein secreted by adipocytes, can stimulate exosome biogenesis in certain cell types [38][51]. Glucagon has been found to regulate exosome production in endothelial cells in adipose tissues [38][52]. The interaction between the exosome biogenesis pathway and the other molecular pathways involved in intracellular vesicle trafficking can complicate the interpretation of functional studies. Different cell types, culture conditions, and cell health can also affect the regulation of exosome biogenesis [20][53]. Inconsistencies in identifying regulatory elements may arise from variations in the methods used for exosome production, enrichment, and concentration [20][54].
The specific case of exosome biogenesis in human syncytiotrophoblasts has been studied at the ultrastructural level. Certain molecules, such as MICA/B, ULBP 1–5, FasL, TRAIL, and PD-L1, are present on the membranes of MVBs and nanosized vesicles within MVBs, but not on the plasma membrane of syncytiotrophoblasts. These molecules may be sorted from the Golgi apparatus to the MVBs. MICA and MICB are expressed on the apical membrane of syncytiotrophoblasts and within exosomes contained within MVBs [35][55].
ESCRTs are not only involved in exosome release but also play a role in packaging biomolecules into exosomes [56]. Heparanase modulates the syndecan–syntenin–ALIX pathway, leading to endosomal membrane budding and subsequent exosome formation by trimming heparan sulfate chains on syndecans [49][56]. However, previous research indicated that cargo molecules within exosomes are segregated into distinct subdomains on the endosomal membrane [56][57]. Additionally, the transfer of exosome-associated domains into the endosomal lumen does not rely on ESCRT function but is induced by sphingolipid ceramide. Purified exosomes contain ceramides, and the inhibition of neutral sphingomyelinases reduces exosome release, suggesting the regulatory role of lipids in exosome secretion [56].
Protein sorting within MVBs, the precursors of exosomes, can occur through the ESCRT-dependent and ESCRT-independent pathways. These pathways may work in synergy, and different subpopulations of exosomes may depend on distinct machinery [47][58] (Figure 1).
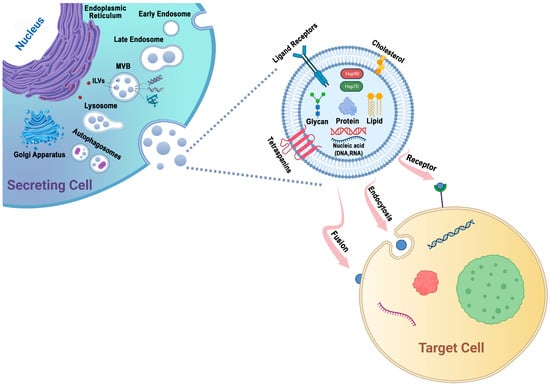
Figure 1. Exosomes are generated through a series of biogenetic processes, starting with the maturation of early endosomes into late endosomes. These late endosomes then transform into MVBs. Within the MVBs, ILVs are formed via the inward budding of the endosomal membrane, which selectively captures proteins, nucleic acids, glycans, and lipids. The MVBs have two main fates: they can fuse with lysosomes for degradation or fuse with the plasma membrane, leading to the release of ILVs as exosomes into the extracellular space. The generation and release of exosomes are influenced by molecules such as tetraspanins and lipids, including cholesterol. These components play important roles in facilitating exosome formation and release. Exosomes possess the capability to adhere to surface receptors present on the target cell, promote endocytosis, or engage in direct fusion with the plasma membrane of the target cell.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines12010123
References
- Ajam-Hosseini, M.; Akhoondi, F.; Doroudian, M. Nano based-oncolytic viruses for cancer therapy. Crit. Rev. Oncol./Hematol. 2023, 185, 103980.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Doroudian, M.; Azhdari, M.H.; Goodarzi, N.; O’Sullivan, D.; Donnelly, S.C. Smart Nanotherapeutics and Lung Cancer. Pharmaceutics 2021, 13, 1972.
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644.
- Shahrivar, R.Y.; Fakhr, Z.A.; Abbasgholinejad, E.; Doroudian, M. Smart Lipid-Based Nanoparticles in Lung Cancer Treatment: Current Status and Future Directions. Adv. Ther. 2023, 6, 2300275.
- Ferro, A.; Sepulcri, M.; Schiavon, M.; Scagliori, E.; Mancin, E.; Lunardi, F.; Gennaro, G.; Frega, S.; Dal Maso, A.; Bonanno, L.; et al. The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments. Cancers 2022, 14, 5700.
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249.
- Laine, A.M.; Westover, K.D.; Choy, H. Radiation Therapy as a Backbone of Treatment of Locally Advanced Non-Small Cell Lung Cancer. Semin. Oncol. 2014, 41, 57–68.
- Petrella, F.; Rizzo, S.; Attili, I.; Passaro, A.; Zilli, T.; Martucci, F.; Bonomo, L.; Del Grande, F.; Casiraghi, M.; De Marinis, F.; et al. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr. Oncol. 2023, 30, 3160–3175.
- Doroudian, M.; Zanganeh, S.; Abbasgholinejad, E.; Donnelly, S. Nanomedicine in Lung Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1144653.
- Jiang, J.; Wang, Y.; Gao, Y.; Sugimura, H.; Minervini, F.; Uchino, J.; Halmos, B.; Yendamuri, S.; Velotta, J.B.; Li, M. Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2022, 11, 277–294.
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol. 2021, 17, 4045–4055.
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, e1413.
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed. Pharmacother. 2020, 128, 110237.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172.
- Nahand, J.S.; Vandchali, N.R.; Darabi, H.; Doroudian, M.; Banafshe, H.R.; Moghoofei, M.; Babaei, F.; Salmaninejad, A.; Mirzaei, H. Exosomal microRNAs: Novel players in cervical cancer. Epigenomics 2020, 12, 1651–1660.
- Hong, C.; Yang, S.; Ndukaife, J.C. Exosomes trapping, manipulation and size-based separation using opto-thermo-electrohydrodynamic tweezers. Nanoscale Adv. 2023, 5, 2973–2978.
- Rajput, A.; Varshney, A.; Bajaj, R.; Pokharkar, V. Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives. Molecules 2022, 27, 7289.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215.
- Hanjani, N.A.; Esmaelizad, N.; Zanganeh, S.; Gharavi, A.T.; Heidarizadeh, P.; Radfar, M.; Omidi, F.; MacLoughlin, R.; Doroudian, M. Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit. Rev. Oncol./Hematol. 2022, 169, 103565.
- Farjadian, F.; Ghasemi, S.; Akbarian, M.; Hoseini-Ghahfarokhi, M.; Moghoofei, M.; Doroudian, M. Physically stimulus-responsive nanoparticles for therapy and diagnosis. Front. Chem. 2022, 10, 952675.
- Manzoor, T.; Saleem, A.; Farooq, N.; Dar, L.A.; Nazir, J.; Saleem, S.; Ismail, S.; Gugjoo, M.B.; Shiekh, P.A.; Ahmad, S.M. Extracellular vesicles derived from mesenchymal stem cells—A novel therapeutic tool in infectious diseases. Inflamm. Regen. 2023, 43, 17.
- Doroudian, M.; Armstrong, M.E.; Donnelly, S.C. Nano-Based Therapies for Acute and Chronic Lung Diseases. In Biotechnology Applied to Inflammatory Diseases: Cellular Mechanisms and Nanomedicine; Ribeiro de Araujo, D., Carneiro-Ramos, M., Eds.; Springer Nature: Singapore, 2023; pp. 271–286.
- Aheget, H.; Tristán-Manzano, M.; Mazini, L.; Cortijo-Gutierrez, M.; Galindo-Moreno, P.; Herrera, C.; Martin, F.; Marchal, J.A.; Benabdellah, K. Exosome: A New Player in Translational Nanomedicine. J. Clin. Med. 2020, 9, 2380.
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022, 20, 291.
- D’Agnelli, S.; Gerra, M.C.; Bignami, E.; Arendt-Nielsen, L. Exosomes as a new pain biomarker opportunity. Mol. Pain. 2020, 16, 1744806920957800.
- Chen, J.; Li, C.; Li, R.; Chen, H.; Chen, D.; Li, W. Exosomes in HIV infection. Curr. Opin. HIV AIDS 2021, 16, 262–270.
- Yu, T.; Xu, Y.; Ahmad, M.A.; Javed, R.; Hagiwara, H.; Tian, X. Exosomes as a Promising Therapeutic Strategy for Peripheral Nerve Injury. Curr. Neuropharmacol. 2021, 19, 2141–2151.
- Yousefi Dehbidi, M.; Goodarzi, N.; Azhdari, M.H.; Doroudian, M. Mesenchymal stem cells and their derived exosomes to combat COVID-19. Rev. Med. Virol. 2022, 32, e2281.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307.
- Wang, Y.; Xu, F.; Zhong, J.Y.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Yuan, L.Q. Exosomes as Mediators of Cell-to-Cell Communication in Thyroid Disease. Int. J. Endocrinol. 2020, 2020, 4378345.
- Burkova, E.E.; Sedykh, S.E.; Nevinsky, G.A. Human Placenta Exosomes: Biogenesis, Isolation, Composition, and Prospects for Use in Diagnostics. Int. J. Mol. Sci. 2021, 22, 2158.
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177.
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47.
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Investig. 2019, 129, 4041–4049.
- Wang, T.; Li, L.; Hong, W. SNARE proteins in membrane trafficking. Traffic 2017, 18, 767–775.
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525.
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177.
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 2021, 22, 204–220.
- Osaki, M.; Okada, F. Exosomes and Their Role in Cancer Progression. Yonago Acta Med. 2019, 62, 182–190.
- Li, K.L.; Huang, H.Y.; Ren, H.; Yang, X.L. Role of exosomes in the pathogenesis of inflammation in Parkinson’s disease. Neural Regen. Res. 2022, 17, 1898–1906.
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91.
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565.
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208.
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685.
- Roucourt, B.; Meeussen, S.; Bao, J.; Zimmermann, P.; David, G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015, 25, 412–428.
- Friand, V.; David, G.; Zimmermann, P. Syntenin and syndecan in the biogenesis of exosomes. Biol. Cell 2015, 107, 331–341.
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 2018, 3, e99680.
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708.e13.
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17.
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738.
- Mincheva-Nilsson, L.; Baranov, V. The role of placental exosomes in reproduction. Am. J. Reprod. Immunol. 2010, 63, 520–533.
- Shan, X.; Zhang, C.; Mai, C.; Hu, X.; Cheng, N.; Chen, W.; Peng, D.; Wang, L.; Ji, Z.; Xie, Y. The Biogenesis, Biological Functions, and Applications of Macrophage-Derived Exosomes. Front. Mol. Biosci. 2021, 8, 715461.
- Hunt, S.D.; Townley, A.K.; Danson, C.M.; Cullen, P.J.; Stephens, D.J. Microtubule motors mediate endosomal sorting by maintaining functional domain organization. J. Cell Sci. 2013, 126, 2493–2501.
- Azhdari, M.H.; Goodarzi, N.; Doroudian, M.; MacLoughlin, R. Molecular Insight into the Therapeutic Effects of Stem Cell-Derived Exosomes in Respiratory Diseases and the Potential for Pulmonary Delivery. Int. J. Mol. Sci. 2022, 23, 6273.
This entry is offline, you can click here to edit this entry!
