Fungi comprise the largest kingdom of higher organisms on the planet: eukaryotes with complex cell structures and abilities to make tissues and organs. Hyphae filaments have a rigid, complex cell wall and moving protoplasm (cytosol) divided into compartments by cross walls termed septa, allowing cellular components to move through these. The plasma membrane comprises a phospholipid bilayer associated with transmembrane proteins and ergosterol and some enzymes such as integral membrane proteins chitin synthase and glucan synthase. The release of enzymes into the extracellular environment, which many fungal species carry out, and the high contact area between filamentous fungi and the soil make these organisms promising for the degradation or immobilization of pollutants (explosives, metals, metalloids, radionuclides, and herbicides) in soil impacted by War-like activities.
- fungi
- bioremediation
- explosives
- radionuclides
- toxic elements
1. Mycoremediation and Its Techniques
“When looking for nature-based solutions to some of our most critical global challenges, fungi could provide many of the answers.” (State of the World’s Fungi 2018 by Katherine Willis, Director of Science, Royal Botanic Gardens, Kew)
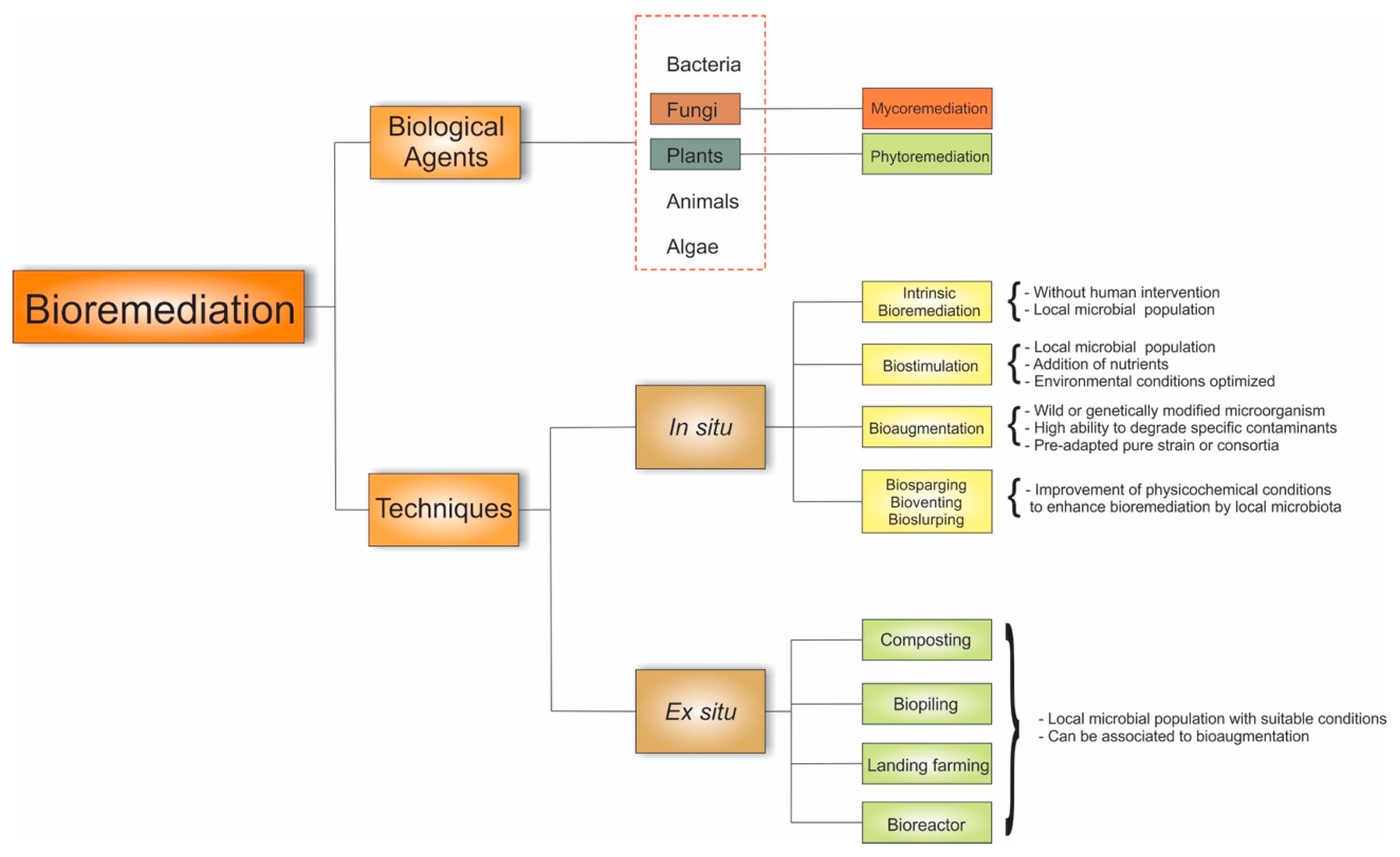
2. Mycoremediation Mechanisms
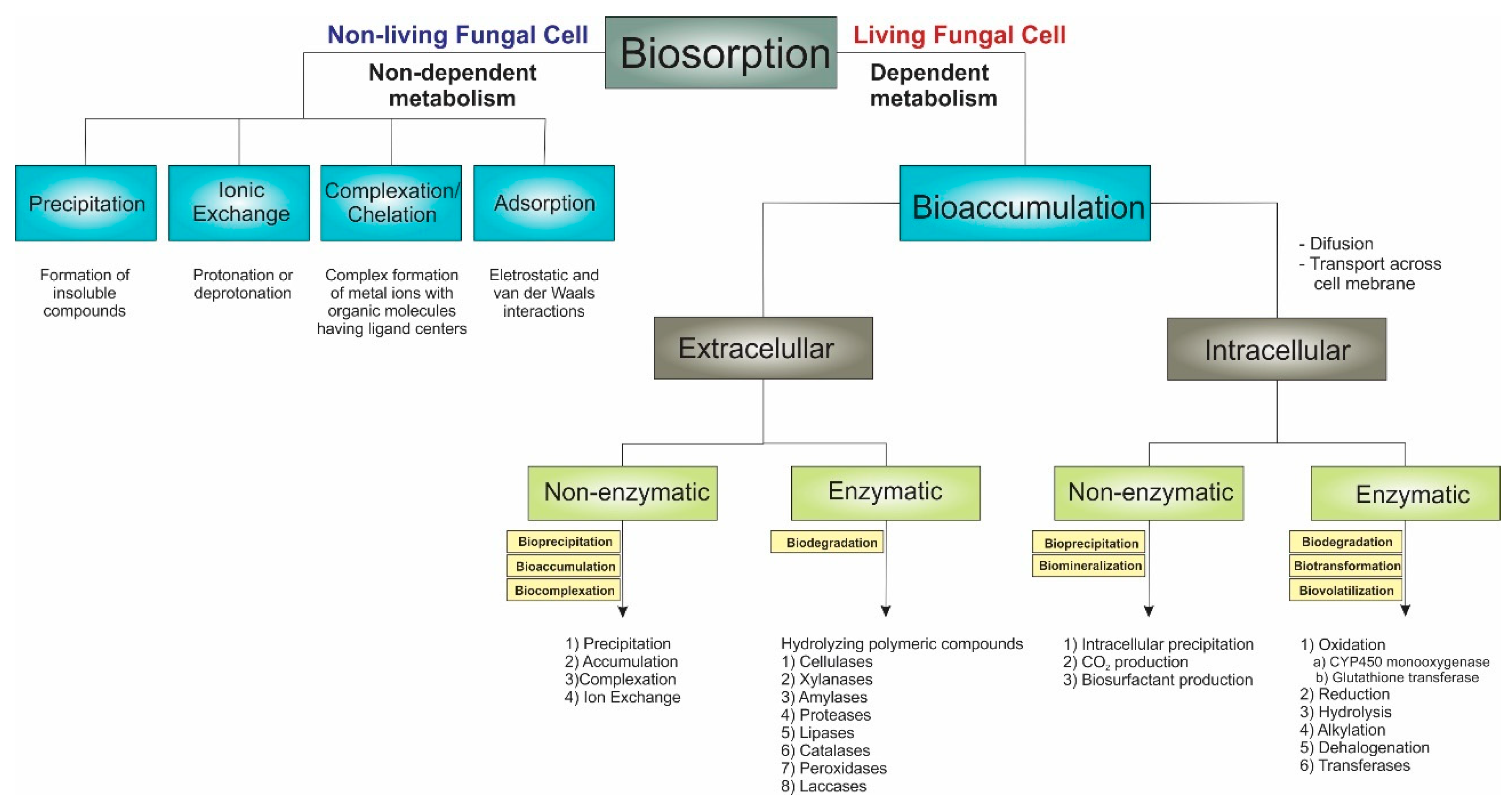
2.1. Fungal Biosorption
- (a)
-
Physical adsorption: functional groups in the cell wall interact electrostatically and through van der Waals forces with pollutants.
- (b)
-
Precipitation: precipitation or solidification is the process of transforming, for example, the toxic metal compounds into their precipitate form, which is less poisonous and almost negligible [48].
- (c)
-
Ion exchange: based on the ion exchange mechanism between the sorbent and the studied pollutants through the replacement (exchange) of protons from the exchangeable sites present on the biosorbent surface with contaminants (e.g., metal ions); this mechanism is facilitated by the existence of hydroxyl, carboxyl, and phenols groups [44].
- (d)
-
Complexation: functional groups in the cell wall provide the ligand atoms necessary to form complexes with metal ions, which attract and retain metals in the biomass [5]. The formation of surface complexes involves the interaction of pollutants (e.g., metal ions) with oxygen donor atoms from the oxygen-containing functional groups (coordination) [5].
2.2. Fungal Bioaccumulation
Fungal Biodegradation and Biotransformation
This entry is adapted from the peer-reviewed paper 10.3390/jof10020094
References
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse Metabolic Capacities of Fungi for Bioremediation. Indian J. Microbiol. 2016, 56, 247–264.
- Wild, J.R.; Varfolomeyev, S.D.; Scozzafava, A. (Eds.) Perspectives in Bioremediation; Springer: Dordrecht, The Netherlands, 1997; pp. 1–12.
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484.
- Singh, H. Mycoremediation: Fungal Bioremediation, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; pp. 29–75.
- Akpasi, S.O.; Anekwe, I.M.S.; Tetteh, E.K.; Amune, U.O.; Shoyiga, H.O.; Mahlangu, T.P.; Kiambi, S.L. Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review. Appl. Sci. 2023, 13, 4978.
- Akhtar, N.; Amin-ul Mannan, M. Mycoremediation: Expunging environmental pollutants. Biotechnol. Rep. 2020, 26, e00452.
- Crocker, F.H.; Jung, C.M.; Indest, K.J.; Everman, S.J.; Carr, M.R. Effects of chitin and temperature on sub-Arctic soil microbial and fungal communities and biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and 2,4-dinitrotoluene (DNT). Biodegradation 2019, 30, 415–431.
- Rasanpreet, K.; Gupta, S.; Tripathi, V.; Chauhan, A.; Parashar, D.; Shankar, P.; Vivek, K. Microbiome based approaches for the degradation of polycyclic aromatic hydrocarbons (PAHs): A current perception. Chemosphere 2023, 341, 139951.
- Treu, R.; Falandysz, J. Mycoremediation of hydrocarbons with basidiomycetes—A review. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2017, 52, 148–155.
- Dickson, U.J.; Coffey, M.; Mortimer, R.J.G.; Di Bonito, M.; Ray, N. Mycoremediation of petroleum contaminated soils: Progress, prospects and perspectives. Environ. Sci. Process. Impacts 2019, 21, 1446–1458.
- Myazin, V.A.; Korneykova, M.V.; Chaporgina, A.A.; Fokina, N.V.; Vasilyeva, G.K. The Effectiveness of Biostimulation, Bioaugmentation and Sorption-Biological Treatment of Soil Contaminated with Petroleum Products in the Russian Subarctic. Microorganisms 2021, 9, 1722.
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Alcántara, C.; Nägele, N.; Antón-Herrero, R.; Escolastico, C.; Eymar, E. Mycoremediation of Soils Polluted with Trichloroethylene: First Evidence of Pleurotus Genus Effectiveness. Appl. Sci. 2021, 11, 1354.
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192.
- Germain, J.; Raveton, M.; Binet, M.N.; Mouhamadou, B. Potentiality of Native Ascomycete Strains in Bioremediation of Highly Polychlorinated Biphenyl Contaminated Soils. Microorganisms 2021, 9, 612.
- Pradeep, S.; Benjamin, S. Mycelial fungi completely remediate di(2-ethylhexyl)phthalate, the hazardous plasticizer in PVC blood storage bag. J. Hazard. Mater. 2012, 235, 69–77.
- Kaewlaoyoong, A.; Chen, J.R.; Cheng, C.Y.; Lin, C.; Cheruiyot, N.K.; Sriprom, P. Innovative mycoremediation technique for treating unsterilized PCDD/F-contaminated field soil and the exploration of chlorinated metabolites. Environ. Pollut. 2021, 289, 117869.
- Kaur, P.; Balomajumder, C. Effective mycoremediation coupled with bioaugmentation studies: An advanced study on newly isolated Aspergillus sp. in Type-II pyrethroid-contaminated soil. Environ. Pollut. 2020, 261, 114073.
- Noman, E.; Talip, B.A.; Al-Gheethi, A.; Mohamed, R.; Nagao, H. Decolourisation of dyes in greywater by mycoremediation and mycosorption process of fungi from peatland; primary study. Mater. Today Proc. 2020, 31, 23–30.
- Baran, W.; Adamek, E.; Wlodarczyk, A.; Lazur, J.; Opoka, W.; Muszynska, B. The remediation of sulfonamides from the environment by Pleurotus eryngii mycelium. Efficiency, products and mechanisms of mycodegradation. Chemosphere 2021, 262, 128026.
- Chakraborty, P.; Abraham, J. Comparative study on degradation of norfloxacin and ciprofloxacin by Ganoderma lucidum JAPC1. Korean J. Chem. Eng. 2017, 34, 1122–1128.
- Ali, A.; Guo, D.; Mahar, A.; Wang, P.; Shen, F.; Li, R.H.; Zhang, Z.Q. Mycoremediation of Potentially Toxic Trace Elements a Biological Tool for Soil Cleanup: A Review. Pedosphere 2017, 27, 205–222.
- Akgul, A.; Ohno, K.M. Mycoremediation of Copper: Exploring the Metal Tolerance of Brown Rot Fungi. Bioresources 2018, 13, 7155–7171.
- Bandurska, K.; Krupa, P.; Berdowska, A.; Jatulewicz, I.; Zawierucha, I. Mycoremediation of soil contaminated with cadmium and lead by Trichoderma sp. Ecol. Chem. Eng. S 2021, 28, 277–286.
- Kumar, V.; Dwivedi, S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412.
- Butnaru, E.; Agoroaci, L.; Mircea, C.; Crivoi, F.; Chinan, V.; Tanase, C.; SGEM. Concentration of metal in mushrooms with potential mycoremediation of soil. In Proceedings of the SGEM 2008: 8th International Scientific Conference, Albena, Bulgaria, 16–20 June 2008; Volume II, pp. 91–98.
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future prospect. Chemosphere 2021, 284, 131325.
- Yadav, P.; Rai, S.N.; Mishra, V.; Singh, M.P. Mycoremediation of environmental pollutants: A review with special emphasis on mushrooms. Environ. Sustain. 2021, 4, 605–618.
- Hassan, A.; Pariatamby, A.; Ahmed, A.; Auta, H.S.; Hamid, F.S. Enhanced Bioremediation of Heavy Metal Contaminated Landfill Soil Using Filamentous Fungi Consortia: A Demonstration of Bioaugmentation Potential. Water Air Soil Pollut. 2019, 230, 215.
- Hassan, A.; Pariatamby, A.; Ossai, I.C.; Hamid, F.S. Bioaugmentation assisted mycoremediation of heavy metal and/metalloid landfill contaminated soil using consortia of filamentous fungi. Biochem. Eng. J. 2020, 157, 107550.
- Pereira, M.d.G.; dos Santos, A.V.; Geris, R.; Malta, M. Chapter 2—Advanced fungal bio-based materials for remediation of toxic metals in aquatic ecosystems. In Novel Materials for Environmental Remediation Applications; Giannakoudakis, D.A., Meili, L., Anastopoulos, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 35–62.
- Kapoor, A.; Viraraghavan, T.; Cullimore, D.R. Removal of heavy metals using the fungus Aspergillus niger. Bioresour. Technol. 1999, 70, 95–104.
- Atlas, R.M.; Philp, J.C. (Eds.) Bioremediation: Applied Microbial Solutions for Real-World Environmental Cleanup; ASM Press: Washington, DC, USA, 2005; p. 165.
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180.
- Carlos, F.S.; Giovanella, P.; Bavaresco, J.; Borges, C.D.; Camargo, F.A.D. A Comparison of Microbial Bioaugmentation and Biostimulation for Hexavalent Chromium Removal from Wastewater. Water Air Soil Pollut. 2016, 227, 175.
- Silva, E.; Fialho, A.M.; Sá-Correia, I.; Burns, R.G.; Shaw, L.J. Combined bioaugmentation and biostimulation to cleanup soil contaminated with high concentrations of atrazine. Environ. Sci. Technol. 2004, 38, 632–637.
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241.
- Davis, M.W.; Glaser, J.A.; Evans, J.W.; Lamar, R.T. Field-Evaluation of the lignin-degrading fungus Phanerochaete-sordida to treat creosote-contaminated soil. Environ. Sci. Technol. 1993, 27, 2572–2576.
- Huang, C.; Zeng, G.M.; Huang, D.L.; Lai, C.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 2017, 243, 294–303.
- Ahtiainen, J.; Valo, R.; Järvinen, M.; Joutti, A. Microbial toxicity tests and chemical analysis as monitoring parameters at composting of creosote-contaminated soil. Ecotoxicol. Environ. Saf. 2002, 53, 323–329.
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytol. 1993, 124, 25–60.
- Volesky, B. Detoxification of metal-bearing effluents: Biosorption for the next century. Hydrometallurgy 2001, 59, 203–216.
- Brazesh, B.; Mousavi, S.M.; Zarei, M.; Ghaedi, M.; Bahrani, S.; Seyyed Alireza, H. Chapter 9—Biosorption. In Adsorption: Fundamental Processes and Applications; Mehrorang, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 587–628.
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly isolated Bacillus sp. PS-6 assisted phytoremediation of heavy metals using Phragmites communis: Potential application in wastewater treatment. Bioresour. Technol. 2021, 320, 124353.
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209.
- Wang, J.L.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226.
- Brito, G.F.M.; Geris, R.; Passos, M.S.; Malta, M.; Ribeiro, J.N.; Licínio, M.; Freitas, D.C.; dos Santos, A.V.; Santos, T.S.M.; Ribeiro, A.; et al. Mycoremediation of Cd2+ and Pb2+ from Aqueous Media by Dead Biomass of Phialomyces macrosporus. Water Air Soil Pollut. 2021, 232, 482.
- Hansda, A.; Kumar, V.; Anshumali. A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170.
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Pham, T.H.T.; Shanmugam, V.; Dragoi, E.N. A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 2023, 312, 137099.
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65.
- Chojnacka, K. Biosorption and bioaccumulation—The prospects for practical applications. Environ. Int. 2010, 36, 299–307.
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973.
- Eccles, H. Removal of heavy-metals from effluent streams—Why select a biological process. Int. Biodeterior. Biodegrad. 1995, 35, 5–16.
- Gadd, G.M. Metals and microorganisms—A problem of definition. FEMS Microbiol. Lett. 1992, 100, 197–203.
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119.
- Hassler, C.S.; Slaveykova, V.I.; Wilkinson, K.J. Discriminating between intra- and extracellular metals using chemical extractions. Limnol. Oceanogr. Methods 2004, 2, 237–247.
- El-Bondkly, A.M.A.; El-Gendy, M. Bioremoval of some heavy metals from aqueous solutions by two different indigenous fungi Aspergillus sp. AHM69 and Penicillium sp. AHM96 isolated from petroleum refining wastewater. Heliyon 2022, 8, e09854.
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Meyer, A.S.; Raj, A. Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci. Total Environ. 2021, 777, 145988.
- Dhagat, S.; Jujjavarapu, S.E. Utility of lignin-modifying enzymes: A green technology for organic compound mycodegradation. J. Chem. Technol. Biotechnol. 2022, 97, 343–358.
- Bumpus, J.A.; Kakar, S.N.; Coleman, R.D. Fungal degradation of organophosphorus inseticides. Appl. Biochem. Biotechnol. 1993, 39, 715–726.
- Lamar, R.T.; Evans, J.W.; Glaser, J.A. Solid-phase treatment of a pentachlorophenol-contaminated soil using lignin-degrading fungi. Environ. Sci. Technol. 1993, 27, 2566–2571.
