In the diverse arsenal of therapeutic tools against cancer, immune checkpoint inhibitors (ICI) have emerged as a new beacon of hope. By inhibiting the immune response’s “OFF” signal, ICIs activate the body’s immune system to attack cancerous growths. Eight immune checkpoint inhibitors have been approved by the FDA for their proven efficacy against multiple cancer types. Per their mechanism of action, ICIs produce a series of well-documented side effects secondary to the induction of immune activation commonly referred to as “immune-related adverse events” (IRAEs). These can affect any organ system, including the eye. Although rare, ocular IRAEs can have debilitating effects on patients’ quality of life and be sight-threatening.
- immune checkpoint inhibitor
- uveitis
- Vogt-Koyanagi-Harada
- birdshot-like uveitis
- immunotherapy
- CTLA-4 inhibitors
- PD-1 inhibitors
- PD-L1 inhibitors
- immune-related adverse events
1. Brief Overview of Immune Checkpoint Inhibitors
1.1. CTLA-4 Inhibitors
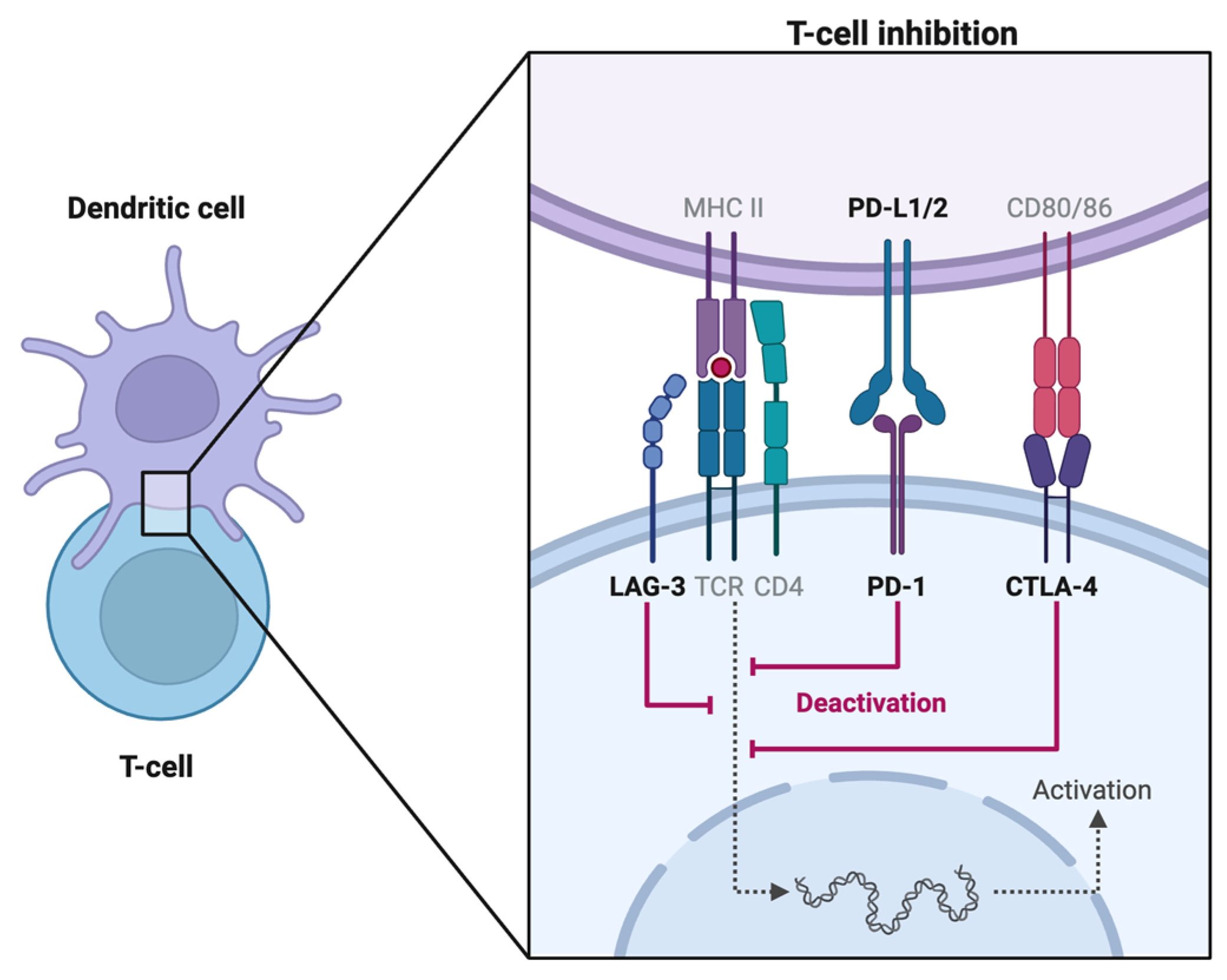
1.2. PD-1 Inhibitors
1.3. PD-L1 Inhibitors
1.4. LAG-3 Inhibitor
2. Side Effects Other Than Ocular Side Effects
|
Organ System |
Reported Adverse Events |
|---|---|
|
Cardiovascular |
Arrhythmia [21] Cardiac failure [22] Hypertension [23] Myocardial infarction [22] Stroke [22] |
|
Endocrine |
Hypothyroidism, hyperthyroidism [26][27] Hyperglycemia [30] |
|
Gastrointestinal/renal |
Colitis/Diarrhea [32] Constipation [26] Esophageal achalasia [33] Hepatitis [34] Nausea [26] Nephritis [35] Pancreatitis [26] |
|
General |
Fatigue [31] Pyrexia [31] |
|
Musculoskeletal |
Inflammatory myopathy [36] Arthropathy [37] |
|
Nervous |
Encephalopathy [20] Facial palsy [38] Hearing loss, vertigo [39][40] Neuropathy [23] |
|
Dermatological |
Alopecia [23] Dermatitis [14] Rash [26] Pruritus [26] Photosensitivity [26] |
|
Respiratory |
Cough [31] Dyspnea [31] Pneumonitis [26] |
|
Other |
Hematological disturbances |
Note: common side effects (those affecting between 1 in 10 and 1 in 100 people) are in bold [14][20].
3. Ocular and Orbital Side Effects Other Than Uveitis
- -
-
Orbit;
- -
-
Anterior segment;
- -
-
Posterior segment
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics14030336
References
- Jago, C.B.; Yates, J.; Olsen Saraiva Câmara, N.; Lechler, R.I.; Lombardi, G. Differential Expression of CTLA-4 among T Cell Subsets. Clin. Exp. Immunol. 2004, 136, 463–471.
- Boyman, O.; Sprent, J. The Role of Interleukin-2 during Homeostasis and Activation of the Immune System. Nat. Rev. Immunol. 2012, 12, 180–190.
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742.
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86.
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological Studies on PD-1 Deficient Mice: Implication of PD-1 as a Negative Regulator for B Cell Responses. Int. Immunol. 1998, 10, 1563–1572.
- Salama, A.D.; Chitnis, T.; Imitola, J.; Ansari, M.J.I.; Akiba, H.; Tushima, F.; Azuma, M.; Yagita, H.; Sayegh, M.H.; Khoury, S.J. Critical Role of the Programmed Death-1 (PD-1) Pathway in Regulation of Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2003, 198, 71–78.
- Mozaffarian, N.; Wiedeman, A.E.; Stevens, A.M. Active Systemic Lupus Erythematosus Is Associated with Failure of Antigen-Presenting Cells to Express Programmed Death Ligand-1. Rheumatol. Oxf. Engl. 2008, 47, 1335–1341.
- Previte, D.M.; Martins, C.P.; O’Connor, E.C.; Marre, M.L.; Coudriet, G.M.; Beck, N.W.; Menk, A.V.; Wright, R.H.; Tse, H.M.; Delgoffe, G.M.; et al. Lymphocyte Activation Gene-3 Maintains Mitochondrial and Metabolic Quiescence in Naive CD4+ T Cells. Cell Rep. 2019, 27, 129–141.e4.
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-Activation Gene 3 (LAG3): The next Immune Checkpoint Receptor. Semin. Immunol. 2019, 42, 101305.
- Puhr, H.C.; Ilhan-Mutlu, A. New Emerging Targets in Cancer Immunotherapy: The Role of LAG3. ESMO Open 2019, 4, e000482.
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-Infiltrating NY-ESO-1-Specific CD8+ T Cells Are Negatively Regulated by LAG-3 and PD-1 in Human Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880.
- Ascione, A.; Arenaccio, C.; Mallano, A.; Flego, M.; Gellini, M.; Andreotti, M.; Fenwick, C.; Pantaleo, G.; Vella, S.; Federico, M. Development of a Novel Human Phage Display-Derived Anti-LAG3 scFv Antibody Targeting CD8+ T Lymphocyte Exhaustion. BMC Biotechnol. 2019, 19, 67.
- OPDUALAG-Nivolumab and Relatlimab-Rmbw Injection. Package Insert. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b22c9d83-3256-4e17-85f7-f331a504adc6 (accessed on 8 October 2023).
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.-E.; Schaeverbeke, T. Immune Related Adverse Events Associated with Anti-CTLA-4 Antibodies: Systematic Review and Meta-Analysis. BMC Med. 2015, 13, 211.
- Weber, J.S.; Antonia, S.J.; Topalian, S.L.; Schadendorf, D.; Larkin, J.M.G.; Sznol, M.; Liu, H.Y.; Waxman, I.; Robert, C. Safety Profile of Nivolumab (NIVO) in Patients (Pts) with Advanced Melanoma (MEL): A Pooled Analysis. J. Clin. Oncol. 2015, 33, 9018.
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients with Advanced Melanoma Receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030.
- Nishino, M.; Sholl, L.M.; Hodi, F.S. Anti–PD-1–Related Pneumonitis during Cancer Immunotherapy. N. Engl. J. Med. 2015, 373, 288–290.
- Ibrahim, R.A.; Berman, D.M.; DePril, V.; Humphrey, R.W.; Chen, T.; Messina, M.; Chin, K.M.; Liu, H.Y.; Bielefield, M.; Hoos, A. Ipilimumab Safety Profile: Summary of Findings from Completed Trials in Advanced Melanoma. J. Clin. Oncol. 2011, 29, 8583.
- Downey, S.G.; Klapper, J.A.; Smith, F.O.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Kammula, U.S.; Hughes, M.S.; Allen, T.E.; Levy, C.L.; et al. Prognostic Factors Related to Clinical Response in Patients with Metastatic Melanoma Treated by CTL-Associated Antigen-4 Blockade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 6681–6688.
- Albandar, H.J.; Fuqua, J.; Albandar, J.M.; Safi, S.; Merrill, S.A.; Ma, P.C. Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers 2021, 13, 989.
- Zimmer, L.; Goldinger, S.M.; Hofmann, L.; Loquai, C.; Ugurel, S.; Thomas, I.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Neurological, Respiratory, Musculoskeletal, Cardiac and Ocular Side-Effects of Anti-PD-1 Therapy. Eur. J. Cancer 2016, 60, 210–225.
- Hu, Y.-B.; Zhang, Q.; Li, H.-J.; Michot, J.M.; Liu, H.-B.; Zhan, P.; Lv, T.-F.; Song, Y. Evaluation of Rare but Severe Immune Related Adverse Effects in PD-1 and PD-L1 Inhibitors in Non-Small Cell Lung Cancer: A Meta-Analysis. Transl. Lung Cancer Res. 2017, 6, S8–S20.
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301.
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757.
- Läubli, H.; Balmelli, C.; Bossard, M.; Pfister, O.; Glatz, K.; Zippelius, A. Acute Heart Failure Due to Autoimmune Myocarditis under Pembrolizumab Treatment for Metastatic Melanoma. J. Immunother. Cancer 2015, 3, 11.
- McArthur, G.A.; Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.V.; et al. Overall Survival (OS) with First-Line Atezolizumab (A) or Placebo (P) in Combination with Vemurafenib (V) and Cobimetinib (C) in BRAFV600 Mutation-Positive Advanced Melanoma: Second Interim OS Analysis of the Phase 3 IMspire150 Study. J. Clin. Oncol. 2022, 40, 9547.
- Min, L.; Hodi, F.S. Anti-PD1 Following Ipilimumab for Mucosal Melanoma: Durable Tumor Response Associated with Severe Hypothyroidism and Rhabdomyolysis. Cancer Immunol. Res. 2014, 2, 15–18.
- Kaehler, K.C.; Egberts, F.; Lorigan, P.; Hauschild, A. Anti-CTLA-4 Therapy-Related Autoimmune Hypophysitis in a Melanoma Patient. Melanoma Res. 2009, 19, 333–334.
- Nallapaneni, N.N.; Mourya, R.; Bhatt, V.R.; Malhotra, S.; Ganti, A.K.; Tendulkar, K.K. Ipilimumab-Induced Hypophysitis and Uveitis in a Patient with Metastatic Melanoma and a History of Ipilimumab-Induced Skin Rash. J. Natl. Compr. Cancer Netw. 2014, 12, 1077–1081.
- Zagouras, A.; Patil, P.D.; Yogi-Morren, D.; Pennell, N.A. Cases from the Immune-Related Adverse Event Tumor Board: Diagnosis and Management of Immune Checkpoint Blockade-Induced Diabetes. Oncologist 2020, 25, 921–924.
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339.
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.L.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in Patients with Cancer after Antibody Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 2283–2289.
- Montes, G.; Duval, F.; Eldani, C.; Amico, S.; Gérard, E.; Dutriaux, C.; Herran, C.; Poullenot, F.; Sole, G.; Carla, L.; et al. Esophageal Achalasia Induced by Ipilimumab and Nivolumab Combination: A Rare Neurological Manifestation of Immune-Related Autonomic Neuropathy. J. Immunother. 2021, 44, 348–350.
- Bernardo, S.G.; Moskalenko, M.; Pan, M.; Shah, S.; Sidhu, H.K.; Sicular, S.; Harcharik, S.; Chang, R.; Friedlander, P.; Saenger, Y.M. Elevated Rates of Transaminitis during Ipilimumab Therapy for Metastatic Melanoma. Melanoma Res. 2013, 23, 47–54.
- Izzedine, H.; Gueutin, V.; Gharbi, C.; Mateus, C.; Robert, C.; Routier, E.; Thomas, M.; Baumelou, A.; Rouvier, P. Kidney Injuries Related to Ipilimumab. Investig. New Drugs 2014, 32, 769–773.
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and Neuromuscular Side-Effects Induced by Immune Checkpoint Inhibitors. Eur. J. Cancer 2019, 106, 12–23.
- de Velasco, G.; Bermas, B.; Choueiri, T.K. Autoimmune Arthropathy and Uveitis as Complications of Programmed Death 1 Inhibitor Treatment. Arthritis Rheumatol. 2016, 68, 556–557.
- Numata, S.; Iwata, Y.; Okumura, R.; Arima, M.; Kobayashi, T.; Watanabe, S.; Suzuki, K.; Horiguchi, M.; Sugiura, K. Bilateral Anterior Uveitis and Unilateral Facial Palsy Due to Ipilimumab for Metastatic Melanoma in an Individual with Human Leukocyte Antigen DR4: A Case Report. J. Dermatol. 2018, 45, 113–114.
- Stürmer, S.H.; Lechner, A.; Berking, C. Sudden Otovestibular Dysfunction in 3 Metastatic Melanoma Patients Treated with Immune Checkpoint Inhibitors. J. Immunother. 2021, 44, 193–197.
- Tampio, A.J.F.; Dhanireddy, S.; Sivapiragasam, A.; Nicholas, B.D. Bilateral Sensorineural Hearing Loss Associated with Nivolumab Therapy for Stage IV Malignant Melanoma. Ear. Nose. Throat J. 2021, 100, 286S–291S.
- Jaber, S.H.; Cowen, E.W.; Haworth, L.R.; Booher, S.L.; Berman, D.M.; Rosenberg, S.A.; Hwang, S.T. Skin Reactions in a Subset of Patients with Stage IV Melanoma Treated with Anti-Cytotoxic T-Lymphocyte Antigen 4 Monoclonal Antibody as a Single Agent. Arch. Dermatol. 2006, 142, 166–172.
- Lise, Q.-K.; Audrey, A.-G. Multifocal Choroiditis as the First Sign of Systemic Sarcoidosis Associated with Pembrolizumab. Am. J. Ophthalmol. Case Rep. 2017, 5, 92–93.
- Murphy, K.P.; Kennedy, M.P.; Barry, J.E.; O’Regan, K.N.; Power, D.G. New-Onset Mediastinal and Central Nervous System Sarcoidosis in a Patient with Metastatic Melanoma Undergoing CTLA4 Monoclonal Antibody Treatment. Oncol. Res. Treat. 2014, 37, 351–353.
- Abdel-Rahman, O.; Oweira, H.; Petrausch, U.; Helbling, D.; Schmidt, J.; Mannhart, M.; Mehrabi, A.; Schöb, O.; Giryes, A. Immune-Related Ocular Toxicities in Solid Tumor Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review. Expert Rev. Anticancer Ther. 2017, 17, 387–394.
- Fortes, B.H.; Liou, H.; Dalvin, L.A. Ophthalmic Adverse Effects of Immune Checkpoint Inhibitors: The Mayo Clinic Experience. Br. J. Ophthalmol. 2021, 105, 1263–1271.
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133.
- Gibney, G.T.; Kudchadkar, R.R.; DeConti, R.C.; Thebeau, M.S.; Czupryn, M.P.; Tetteh, L.; Eysmans, C.; Richards, A.; Schell, M.J.; Fisher, K.J.; et al. Safety, Correlative Markers, and Clinical Results of Adjuvant Nivolumab in Combination with Vaccine in Resected High-Risk Metastatic Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 712–720.
- Goldstein, B.L.; Gedmintas, L.; Todd, D.J. Drug-Associated Polymyalgia Rheumatica/Giant Cell Arteritis Occurring in Two Patients After Treatment with Ipilimumab, an Antagonist of CTLA-4. Arthritis Rheumatol. 2014, 66, 768–769.
- Chang, E.; Sabichi, A.L.; Sada, Y.H. Myasthenia Gravis After Nivolumab Therapy for Squamous Cell Carcinoma of the Bladder. J. Immunother. 2017, 40, 114–116.
- Makarious, D.; Horwood, K.; Coward, J.I.G. Myasthenia Gravis: An Emerging Toxicity of Immune Checkpoint Inhibitors. Eur. J. Cancer 2017, 82, 128–136.
- Papavasileiou, E.; Prasad, S.; Freitag, S.K.; Sobrin, L.; Lobo, A.-M. Ipilimumab-Induced Ocular and Orbital Inflammation--A Case Series and Review of the Literature. Ocul. Immunol. Inflamm. 2016, 24, 140–146.
- Nguyen, A.T.; Elia, M.; Materin, M.A.; Sznol, M.; Chow, J. Cyclosporine for Dry Eye Associated with Nivolumab: A Case Progressing to Corneal Perforation. Cornea 2016, 35, 399.
- Cappelli, L.C.; Gutierrez, A.K.; Baer, A.N.; Albayda, J.; Manno, R.L.; Haque, U.; Lipson, E.J.; Bleich, K.B.; Shah, A.A.; Naidoo, J.; et al. Inflammatory Arthritis and Sicca Syndrome Induced by Nivolumab and Ipilimumab. Ann. Rheum. Dis. 2017, 76, 43–50.
- Baughman, D.M.; Lee, C.S.; Snydsman, B.E.; Jung, H.C. Bilateral Uveitis and Keratitis Following Nivolumab Treatment for Metastatic Melanoma. Med. Case Rep. 2017, 3, 8.
- Golash, V.; Almeida, G. Pembrolizumab-Related Bilateral Ocular Hypotony, Uveitis, Cataracts, Exudative Retinal, and Choroidal Detachments: An Unusual Success Story. J. Immunother. 2020, 43, 283–285.
- Itzam Marin, A.; Deitz, G.A.; Mudie, L.I.; Reddy, A.K.; Palestine, A.G. Bilateral Choroidal Effusions and Angle Closure in the Setting of Systemic Capillary Leak Syndrome from HLA-Directed Vaccine and Pembrolizumab Therapy for Squamous Cell Carcinoma. Am. J. Ophthalmol. Case Rep. 2023, 29, 101777.
- Ahmed, A.A.; Hiya, F.E.; Eichenbaum, D.A. Bilateral Hypotony Maculopathy Associated with Ipilimumab and Nivolumab Therapy Unresponsive to Corticosteroid Treatment. Ophthalmic Surg. Lasers Imaging Retin. 2023, 54, 301–304.
- Funagura, N.; Fukushima, S.; Inoue, T. Ipilimumab-Related Uveitis and Refractory Hypotony with a Flat Chamber in a Trabeculectomized Eye with Exfoliation Glaucoma: A Case Report. Am. J. Ophthalmol. Case Rep. 2023, 29, 101807.
- Lee, J.C.; Al-Humimat, G.; Kooner, K.S. Acute Bilateral Uveitis, Hypotony, and Cataracts Associated with Ipilimumab and Nivolumab Therapy: Optical Coherence Tomography Angiography Findings. Case Rep. Ophthalmol. 2020, 11, 606–611.
- Nguyen, M.; Islam, M.R.; Lim, S.W.; Sahu, A.; Tamjid, B. Pembrolizumab Induced Ocular Hypotony with Near Complete Vision Loss, Interstitial Pulmonary Fibrosis and Arthritis. Front. Oncol. 2019, 9, 944.
- Reid, G.; Lorigan, P.; Heimann, H.; Hovan, M. Management of Chronic Hypotony Following Bilateral Uveitis in a Patient Treated with Pembrolizumab for Cutaneous Metastatic Melanoma. Ocul. Immunol. Inflamm. 2019, 27, 1012–1015.
- Yeh, O.L.; Francis, C.E. Ipilimumab-Associated Bilateral Optic Neuropathy. J. Neuro-Ophthalmol. Off. J. North Am. Neuro-Ophthalmol. Soc. 2015, 35, 144–147.
- Wilson, M.A.; Guld, K.; Galetta, S.; Walsh, R.D.; Kharlip, J.; Tamhankar, M.; McGettigan, S.; Schuchter, L.M.; Fecher, L.A. Acute Visual Loss after Ipilimumab Treatment for Metastatic Melanoma. J. Immunother. Cancer 2016, 4, 66.
- Manusow, J.S.; Khoja, L.; Pesin, N.; Joshua, A.M.; Mandelcorn, E.D. Retinal Vasculitis and Ocular Vitreous Metastasis Following Complete Response to PD-1 Inhibition in a Patient with Metastatic Cutaneous Melanoma. J. Immunother. Cancer 2014, 2, 41.
- Tsui, E.; Gonzales, J.A. Retinal Vasculitis Associated with Ipilimumab. Ocul. Immunol. Inflamm. 2020, 28, 868–870.
- de Vries, E.W.; Schauwvlieghe, A.-S.; Haanen, J.B.; de Hoog, J. Bilateral Serous Retinal Detachment and Uveitis Associated with Pembrolizumab Treatment in Metastatic Melanoma. Retin. Cases Brief Rep. 2022, 16, 430–434.
