Technologies to capture CO2 and use it as a feedstock to produce CO2-based chemicals and biofuels via chemical or biochemical conversion pathways can potentially reduce the amount of CO2 released. Cultivation of microalgae, an intermediate CO2-based product of microalgal biorefineries, is recognized as a promising decentralized alternative to capture and use CO2 emissions using biological transformation. Microalgal strains are generally cultivated in open raceways or closed (flat panel, tubular) cultivation systems. It is generally known that CO2, nutrients (mainly N compounds and phosphates), and sunlight as an energy source are needed for microalgal growth under photosynthetic reaction.
- anaerobic digestion
- carbon capture and use
- energy sustainability
- hydrothermal carbonization
1. Microalgal Cultivation Systems


2. Sustainable Renewable Energy and Green Products from Microalgae
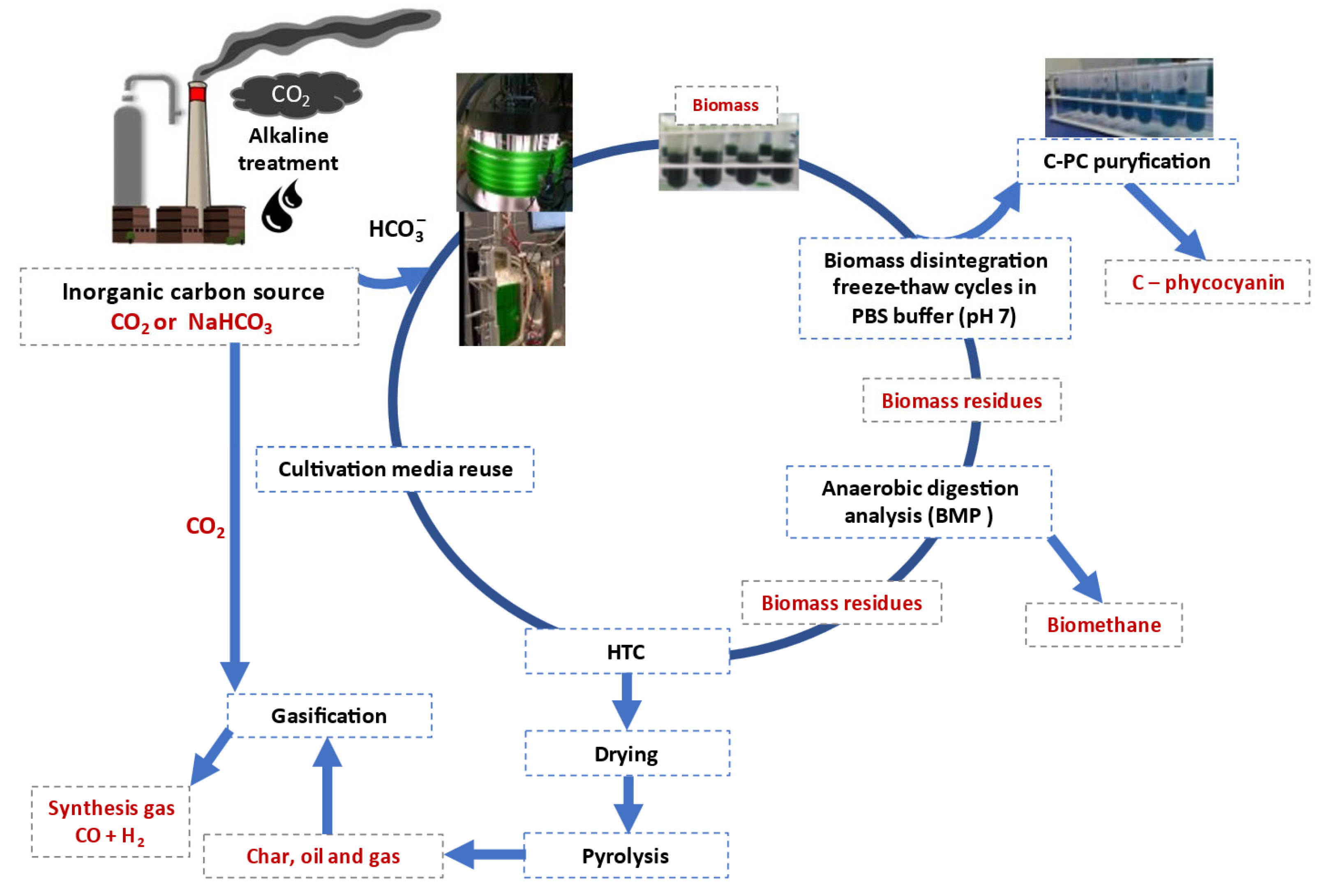
2.1. Anaerobic Fermentation of Microalgal Residues
2.2. Hydrothermal Carbonization of Microalgal Residues
2.3. Pyrolysis of Microalgal Residues
2.4. Gasification of Microalgal Residues in CO2 Atmosphere
3. Biochemical CO2-to-X Sustainability towards LCA Analysis
This entry is adapted from the peer-reviewed paper 10.3390/su16031201
References
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232.
- Wang, B.; Lan, C.Q.; Horsman, M. Closed Photobioreactors for Production of Microalgal Biomasses. Biotechnol. Adv. 2012, 30, 904–912.
- Cheregi, O.; Ekendahl, S.; Engelbrektsson, J.; Strömberg, N.; Godhe, A.; Spetea, C. Microalgae Biotechnology in Nordic Countries—The Potential of Local Strains. Physiol. Plant 2019, 166, 438–450.
- Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 23 November 2023).
- Moody, J.W.; McGinty, C.M.; Quinn, J.C. Global Evaluation of Biofuel Potential from Microalgae. Proc. Natl. Acad. Sci. USA 2014, 111, 8691–8696.
- Kratky, L.; Jirout, T.; Belohlav, V. Economic Feasibility Study for Artificial Lighting of Microalgal Flat-Panel Photobioreactors. Int. J. Environ. Sci. Technol. 2023, 20, 12089–12100.
- Bitog, J.P.; Lee, I.-B.; Lee, C.-G.; Kim, K.-S.; Hwang, H.-S.; Hong, S.-W.; Seo, I.-H.; Kwon, K.-S.; Mostafa, E. Application of Computational Fluid Dynamics for Modeling and Designing Photobioreactors for Microalgae Production: A Review. Comput. Electron. Agric. 2011, 76, 131–147.
- Lyon, B.R.; Mock, T. Polar Microalgae: New Approaches towards Understanding Adaptations to an Extreme and Changing Environment. Biology 2014, 3, 56–80.
- Hulatt, C.J.; Berecz, O.; Egeland, E.S.; Wijffels, R.H.; Kiron, V. Polar Snow Algae as a Valuable Source of Lipids? Bioresour. Technol. 2017, 235, 338–347.
- León-Vaz, A.; León, R.; Vigara, J.; Funk, C. Exploring Nordic Microalgae as a Potential Novel Source of Antioxidant and Bioactive Compounds. New Biotechnol. 2023, 73, 1–8.
- Cicci, A.; Stoller, M.; Bravi, M. Analysis of Microalgae Growth in Residual Light: A Diagnostics Tool for Low-Cost Alternative Cultural Media. Chem. Eng. Trans. 2014, 38, 79–84.
- Bělohlav, V.; Jirout, T.; Elster, J.; Liška, J.; Nedbalová, L.; Kvíderová, J. Cultivation of Polar Microalgae in a Rotating Flat-Panel Photobioreactor. Chem. Listy 2023, 117, 613–618.
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to Produce Cost-Effective Third-Generation Biofuel from Microalgae. Front. Energy Res. 2021, 9, 749968.
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666.
- Algalif in the Media. Available online: https://algalif.is/category/algalif-in-the-media/ (accessed on 23 November 2023).
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429.
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592.
- Gluszcz, P.; Klepacz-Smółka, A.; Ledakowicz, S. Experimental Evaluation of a Helical Laboratory Photobioreactor for Cultivation of Thermophilic Cyanobacteria—Hydrodynamics and Mass Transfer Studies. Chem. Process Eng.-Inz. Chem. Proces. 2018, 39, 457–473.
- Heubeck, S.; Craggs, R.J.; Shilton, A. Influence of CO2 Scrubbing from Biogas on the Treatment Performance of a High Rate Algal Pond. Water Sci. Technol. 2007, 55, 193–200.
- Antecka, A.; Klepacz-Smółka, A.; Szeląg, R.; Pietrzyk, D.; Ledakowicz, S. Comparison of Three Methods for Thermostable C-Phycocyanin Separation and Purification. Chem. Eng. Process. Process Intensif. 2022, 171, 108563.
- Ledakowicz, S.; Slezak, R. From Biofuels to High Added Value Bioproducts in the Development of 3G Biorefineries. Available online: https://ccuv4.fs.cvut.cz/events/the-ccuv4-workshop-in-prague/ (accessed on 16 January 2024).
- Klepacz-Smolka, A.; Shah, M.R.; Jiang, Y.; Zhong, Y.; Chen, P.; Pietrzyk, D.; Szelag, R.; Ledakowicz, S.; Daroch, M. Microalgae Are Not an Umbrella Solution for Power Industry Waste Abatement but Could Play a Role in Their Valorization. Crit. Rev. Biotechnol. 2023, 1–29.
- Abusweireh, R.S.; Rajamohan, N.; Sonne, C.; Vasseghian, Y. Algae Biogas Production Focusing on Operating Conditions and Conversion Mechanisms—A Review. Heliyon 2023, 9, e17757.
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic Digestion of Algae Biomass: A Review. Algal Res. 2014, 5, 204–214.
- Wang, Y.; Wei, W.; Huang, Q.-S.; Ni, B.-J. Methane Production from Algae in Anaerobic Digestion: Role of Corncob Ash Supplementation. J. Clean. Prod. 2021, 327, 129485.
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation Coupled Production of Algal Biomass, Harvesting and Anaerobic Digestion: Possibilities and Challenges. Biotechnol. Adv. 2013, 31, 1408–1425.
- Akizuki, S.; Cuevas-Rodríguez, G.; Toda, T. Effect of Ammonia Concentration on a Microalgal-Nitrifying Bacterial Photobioreactor Treating Anaerobic Digester Effluent. Biochem. Eng. J. 2021, 173, 108057.
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503.
- Ferreira, L.O.; Astals, S.; Passos, F. Anaerobic Co-digestion of Food Waste and Microalgae in an Integrated Treatment Plant. J. Chem. Technol. Biotechnol. 2022, 97, 1545–1554.
- Park, H.J.; Heo, H.S.; Jeon, J.-K.; Kim, J.; Ryoo, R.; Jeong, K.-E.; Park, Y.-K. Highly Valuable Chemicals Production from Catalytic Upgrading of Radiata Pine Sawdust-Derived Pyrolytic Vapors over Mesoporous MFI Zeolites. Appl. Catal. B 2010, 95, 365–373.
- Bahrun, M.H.V.; Bono, A.; Othman, N.; Zaini, M.A.A. Carbon Dioxide Removal from Biogas through Pressure Swing Adsorption—A Review. Chem. Eng. Res. Des. 2022, 183, 285–306.
- Yan, S.; He, Q.; Zhao, S.; Wang, Y.; Ai, P. Biogas Upgrading by CO2 Removal with a Highly Selective Natural Amino Acid Salt in Gas–Liquid Membrane Contactor. Chem. Eng. Process. Process Intensif. 2014, 85, 125–135.
- Koutsiantzi, C.; Kampylafka, A.; Zouboulis, A.; Mitrakas, M.; Kikkinides, E.S. Theoretical and Experimental Study of CO2 Removal from Biogas Employing a Hollow Fiber Polyimide Membrane. Sustain. Chem. Pharm. 2023, 35, 101221.
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283.
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in Microalgae Application for CO2 Sequestration. Clean. Chem. Eng. 2022, 3, 100044.
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056.
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Integration of Anaerobic Digestion and Microalgal Cultivation for Digestate Bioremediation and Biogas Upgrading. Bioresour. Technol. 2019, 290, 121804.
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic Digestate as a Low-Cost Nutrient Source for Sustainable Microalgae Cultivation: A Way Forward through Waste Valorization Approach. Sci. Total Environ. 2022, 803, 150070.
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of Characterization and Adsorption of Biochars Produced from Hydrothermal Carbonization and Pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35.
- Kozyatnyk, I.; Benavente, V.; Weidemann, E.; Gentili, F.G.; Jansson, S. Influence of Hydrothermal Carbonization Conditions on the Porosity, Functionality, and Sorption Properties of Microalgae Hydrochars. Sci. Rep. 2023, 13, 8562.
- de Siqueira Castro, J.; Assemany, P.P.; de Oliveira Carneiro, A.C.; Ferreira, J.; de Jesus Júnior, M.M.; de Ávila Rodrigues, F.; Calijuri, M.L. Hydrothermal Carbonization of Microalgae Biomass Produced in Agro-Industrial Effluent: Products, Characterization and Applications. Sci. Total Environ. 2021, 768, 144480.
- Heilmann, S.M.; Jader, L.R.; Harned, L.A.; Sadowsky, M.J.; Schendel, F.J.; Lefebvre, P.A.; von Keitz, M.G.; Valentas, K.J. Hydrothermal Carbonization of Microalgae II. Fatty Acid, Char, and Algal Nutrient Products. Appl. Energy 2011, 88, 3286–3290.
- García-Bordejé, E.; Pires, E.; Fraile, J.M. Parametric Study of the Hydrothermal Carbonization of Cellulose and Effect of Acidic Conditions. Carbon 2017, 123, 421–432.
- Ślęzak, R.; Nawrot, P.; Ledakowicz, S. Pyrolysis of Micro- and Macroalgae in Thermobalance Coupled with Mass Spectrometer. Algal Res. 2022, 66, 102782.
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass Fast Pyrolysis in a Fluidized Bed Reactor under N2, CO2, CO, CH4 and H2 Atmospheres. Bioresour. Technol. 2011, 102, 4258–4264.
- Kwon, E.E.; Jeon, Y.J.; Yi, H. New Candidate for Biofuel Feedstock beyond Terrestrial Biomass for Thermo-Chemical Process (Pyrolysis/Gasification) Enhanced by Carbon Dioxide (CO2). Bioresour. Technol. 2012, 123, 673–677.
- Hong, Y.; Xie, C.; Chen, W.; Luo, X.; Shi, K.; Wu, T. Kinetic Study of the Pyrolysis of Microalgae under Nitrogen and CO2 Atmosphere. Renew. Energy 2020, 145, 2159–2168.
- Díaz-Rey, M.R.; Cortés-Reyes, M.; Herrera, C.; Larrubia, M.A.; Amadeo, N.; Laborde, M.; Alemany, L.J. Hydrogen-Rich Gas Production from Algae-Biomass by Low Temperature Catalytic Gasification. Catal. Today 2015, 257, 177–184.
- Slezak, R.; Unyay, H.; Szufa, S.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Reactors Torrefaction vs. Biomass Pyrolizers—Part 2. Energies 2023, 16, 2212.
- Hong, Y.; Chen, W.; Luo, X.; Pang, C.; Lester, E.; Wu, T. Microwave-Enhanced Pyrolysis of Macroalgae and Microalgae for Syngas Production. Bioresour. Technol. 2017, 237, 47–56.
- Wu, C.; Budarin, V.L.; Wang, M.; Sharifi, V.; Gronnow, M.J.; Wu, Y.; Swithenbank, J.; Clark, J.H.; Williams, P.T. CO2 Gasification of Bio-Char Derived from Conventional and Microwave Pyrolysis. Appl. Energy 2015, 157, 533–539.
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072.
- Xiao, R.; Yang, W. Influence of Temperature on Organic Structure of Biomass Pyrolysis Products. Renew. Energy 2013, 50, 136–141.
- Xu, W.; Ding, K.; Hu, L. A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals. Processes 2021, 9, 2042.
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic Pyrolysis of Microalgae to High-Quality Liquid Bio-Fuels. Biomass Bioenergy 2011, 35, 3199–3207.
- Slezak, R.; Krzystek, L.; Ledakowicz, S. CO2 Gasification of Char from Spent Mushroom Substrate in TG-MS System. J. Therm. Anal. Calorim. 2020, 140, 2337–2345.
- Tanner, J.; Bhattacharya, S. Kinetics of CO2 and Steam Gasification of Victorian Brown Coal Chars. Chem. Eng. J. 2016, 285, 331–340.
- Liu, L.; Cao, Y.; Liu, Q.; Yang, J. Experimental and Kinetic Studies of Coal–CO2 Gasification in Isothermal and Pressurized Conditions. RSC Adv. 2017, 7, 2193–2201.
- Wang, L.; Alsaker, N.; Skreiberg, Ø.; Hovd, B. Effect of Carbonization Conditions on CO2 Gasification Reactivity of Biocarbon. Energy Procedia 2017, 142, 932–937.
- Bui, H.-H.; Wang, L.; Tran, K.-Q.; Skreiberg, Ø. CO2 Gasification of Charcoals Produced at Various Pressures. Fuel Process. Technol. 2016, 152, 207–214.
- Wang, F.; Zeng, X.; Shao, R.; Wang, Y.; Yu, J.; Xu, G. Isothermal Gasification of in Situ/Ex Situ Coal Char with CO2 in a Micro Fluidized Bed Reaction Analyzer. Energy Fuels 2015, 29, 4795–4802.
- Atikah, M.S.N.; Taufiq Yap, Y.H.; Ilyas, R.A.; Harun, R. Optimization of Algae Residues Gasification: Experimental and Theoretical Approaches. J. Phys. Conf. Ser. 2022, 2259, 012012.
- Roncancio, R.; Gore, J.P. CO2 Char Gasification: A Systematic Review from 2014 to 2020. Energy Convers. Manag. X 2021, 10, 100060.
- Pohořelý, M.; Jeremiáš, M.; Svoboda, K.; Kameníková, P.; Skoblia, S.; Beňo, Z. CO2 as Moderator for Biomass Gasification. Fuel 2014, 117, 198–205.
- Massoudi Farid, M.; Jeong, H.J.; Hwang, J. Co-Gasification of Coal–Biomass Blended Char with CO2 and H2O: Effect of Partial Pressure of the Gasifying Agent on Reaction Kinetics. Fuel 2015, 162, 234–238.
- Gao, N.; Śliz, M.; Quan, C.; Bieniek, A.; Magdziarz, A. Biomass CO2 Gasification with CaO Looping for Syngas Production in a Fixed-Bed Reactor. Renew. Energy 2021, 167, 652–661.
- Grenz, J.; Cerdas, F.; Herrmann, C. LCA Based Analysis of Product Portfolios—Towards Decarbonization. Procedia CIRP 2022, 105, 519–524.
- Intergovernmental Panel on Climate Change (IPCC) (Ed.) Climate Change 2022—Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2023; ISBN 9781009157926.
- Bach, V. Life Cycle Assessment in the Context of Decarbonization and Carbon Neutrality. Int. J. Life Cycle Assess. 2023, 28, 741–745.
- de Souza Schneider, R.D.C.; de Moura Lima, M.; Hoeltz, M.; de Farias Neves, F.; John, D.K.; de Azevedo, A. Life Cycle Assessment of Microalgae Production in a Raceway Pond with Alternative Culture Media. Algal Res. 2018, 32, 280–292.
- Porcelli, R.; Dotto, F.; Pezzolesi, L.; Marazza, D.; Greggio, N.; Righi, S. Comparative Life Cycle Assessment of Microalgae Cultivation for Non-Energy Purposes Using Different Carbon Dioxide Sources. Sci. Total Environ. 2020, 721, 137714.
- Bradley, T.; Rajaeifar, M.A.; Kenny, A.; Hainsworth, C.; del Pino, V.; del Valle Inclán, Y.; Povoa, I.; Mendonça, P.; Brown, L.; Smallbone, A.; et al. Life Cycle Assessment of Microalgae-Derived Biodiesel. Int. J. Life Cycle Assess. 2023, 28, 590–609.
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of the Red Antioxidant Carotenoid Astaxanthin by Microalgae: From Lab to Pilot Scale. J. Clean. Prod. 2014, 64, 332–344.
- López-Herrada, E.; Gallardo-Rodríguez, J.J.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F. Life-Cycle Assessment of a Microalgae-Based Fungicide under a Biorefinery Approach. Bioresour. Technol. 2023, 383, 129244.
- Sangma, C.B.K.; Chalie-u, R. Life Cycle Assessment of Wastewater Treatment by Microalgae. In Valorization of Microalgal Biomass and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–178.
- Lu, Y.; Mu, D.; Xue, Z.; Xu, P.; Li, Y.; Xiang, W.; Burnett, J.; Bryant, K.; Zhou, W. Life Cycle Assessment of Industrial Production of Microalgal Oil from Heterotrophic Fermentation. Algal Res. 2021, 58, 102404.
- Wimmerova, L.; Keken, Z.; Solcova, O.; Vavrova, K. A Comparative Analysis of Environmental Impacts of Operational Phases of Three Selected Microalgal Cultivation Systems. Sustainability 2022, 15, 769.
- Huang, X.; Bai, S.; Liu, Z.; Hasunuma, T.; Kondo, A.; Ho, S.-H. Fermentation of Pigment-Extracted Microalgal Residue Using Yeast Cell-Surface Display: Direct High-Density Ethanol Production with Competitive Life Cycle Impacts. Green Chem. 2020, 22, 153–162.
- Sun, C.; Xia, A.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X. Life-Cycle Assessment of Biohythane Production via Two-Stage Anaerobic Fermentation from Microalgae and Food Waste. Renew. Sustain. Energy Rev. 2019, 112, 395–410.
- Ubando, A.T.; Rivera, D.R.T.; Chen, W.-H.; Culaba, A.B. A Comprehensive Review of Life Cycle Assessment (LCA) of Microalgal and Lignocellulosic Bioenergy Products from Thermochemical Processes. Bioresour. Technol. 2019, 291, 121837.
- Fortier, M.-O.P.; Roberts, G.W.; Stagg-Williams, S.M.; Sturm, B.S.M. Life Cycle Assessment of Bio-Jet Fuel from Hydrothermal Liquefaction of Microalgae. Appl. Energy 2014, 122, 73–82.
- Naaz, F.; Samuchiwal, S.; Dalvi, V.; Bhattacharya, A.; Kishore Pant, K.; Malik, A. Hydrothermal Liquefaction Could Be a Sustainable Approach for Valorization of Wastewater Grown Algal Biomass into Cleaner Fuel. Energy Convers. Manag. 2023, 283, 116887.
- Chen, P.H.; Quinn, J.C. Microalgae to Biofuels through Hydrothermal Liquefaction: Open-Source Techno-Economic Analysis and Life Cycle Assessment. Appl. Energy 2021, 289, 116613.
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of Microalgae: A Critical Review. Fuel Process. Technol. 2019, 186, 53–72.
- Grierson, S.; Strezov, V.; Bengtsson, J. Life Cycle Assessment of a Microalgae Biomass Cultivation, Bio-Oil Extraction and Pyrolysis Processing Regime. Algal Res. 2013, 2, 299–311.
- Wang, X.; Guo, F.; Li, Y.; Yang, X. Effect of Pretreatment on Microalgae Pyrolysis: Kinetics, Biocrude Yield and Quality, and Life Cycle Assessment. Energy Convers. Manag. 2017, 132, 161–171.
- Azadi, P.; Brownbridge, G.; Mosbach, S.; Inderwildi, O.; Kraft, M. Simulation and Life Cycle Assessment of Algae Gasification Process in Dual Fluidized Bed Gasifiers. Green Chem. 2015, 17, 1793–1801.
