Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Medicinal
The mannose receptor (MR, CD 206) is an endocytic receptor primarily expressed by macrophages and dendritic cells, which plays a critical role in both endocytosis and antigen processing and presentation. MR carbohydrate recognition domains (CRDs) exhibit a high binding affinity for branched and linear oligosaccharides. Furthermore, multivalent mannose presentation on the various templates like peptides, proteins, polymers, micelles, and dendrimers was proven to be a valuable approach for the selective and efficient delivery of various therapeutically active agents to MR.
- mannose
- mannose receptor
- ligand–receptor interaction
- targeted drug delivery
1. Mannose-Based Glycomimetics as Ligands for MR
A large variety of multivalent platforms were employed as cores for mannose-mediated MR presentation, including liposomes, peptides, proteins, various polymers, micelles, dendrimers, dendrons, and nanoparticles. Sugars have good targeting specificity, low toxicity, and biocompatibility. With the development of glycobiology, sugar chain engineering and glycomics, the application of sugars in medicine was developed quickly [74]. These sugar structures achieve the effective and active targeting of various tissues and cells and have the ability to load different kinds of drugs, genes, and therapeutic RNA molecules [75]. Recently, an important study of trimannose-coupling for effective delivery of inhaled oligonucleotides to pulmonary macrophages was published as the first mannosylated therapeutic candidate for COVID-19 [76]. Lately, mannose is acquiring more and more interest as a promising target ligand in cancer therapy.
High-mannose structures that cover the surface of many pathogenic microorganisms are readily recognized by the MR CRDs. All complex mannose glycans share the common pentasaccharide core Manα(1-6)[Manα(1-3)]Manβ(1-4)GlcNAcβ(1-4)GlcNAc (Man3GlcNAc2) carrying on to six additional mannose residues (Figure 2A). Mannan is a polysaccharide of mannose found in the cell wall of Saccharomyces cerevisiae (yeast) that binds to the MR CRDs and causes an immune response involving the up-regulation of co-stimulatory molecules and pro-inflammatory cytokines [12]. Due to this ability, some vaccine design attempts that mimic yeast mannose cell walls were designed. In addition, studies with synthetic mucins and some other synthetic glycopeptides that mimic natural oligomannosides were carried out in order to investigate how mannose spacing and valency affect the lectin recognition and mode of binding [77,78]. In addition, a family of branched mannooligosaccharide mimics of natural compounds, prepared by exploiting copper(I)-catalyzed azide-alkyne cycloaddition ligation strategy, was demonstrated, and their binding affinities towards the human macrophage MR (rhMMR) was examined by François-Heude and co-workers [79]. The authors replaced the demanding internal mannopyranosyl units with triazole rings while retaining the terminal mannose display (Figure 2B). The binding potency of the new pseudo-Man derivatives to concanavalin A and rhMMR was comparable to that of their respective natural partners [79,80].
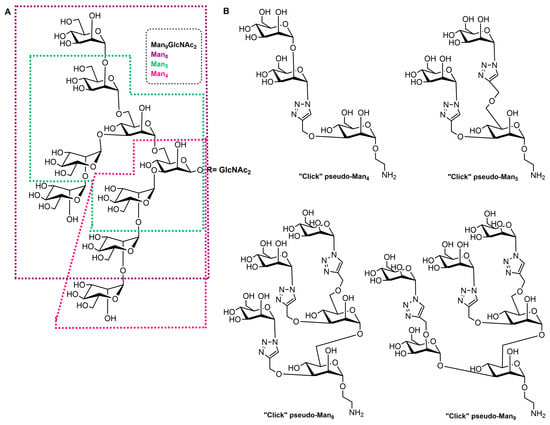
Figure 2. (A) Structure of the Man9GlcNAc2 oligosaccharide with the Man4, Man5, and Man8 substructures highlighted with appropriate colors. (B) Structures of the “click” pseudo-high-mannose mimics.
For the past four decades, several other groups reported the synthesis of such structures; anyway, the preparation of high-mannose structures remains very challenging, as well as efficient incorporation into delivery systems. In general, the synthesis of multivalent mannosylated conjugates requires the attachment of mannose or its appropriate derivative (natural or synthetic ones) on a functionalized scaffold. Mono-, di-, oligo-mannose, or a polymer of mannose (mannan) are usually used in mannosylation reactions. Conventionally, the coupling procedure is commonly initiated by the amine-activated carboxylic acid reaction, copper-catalyzed click reactions, and, lately, the copper-free click-based strategy [13,18,26,27,79,80]. Apart from covalent synthetic strategies, the coupling of a ligand to a nanoparticle can be accomplished by electrostatic interactions [81,82].
Among non-covalent nanoparticle bioconjugation strategies, the spontaneous self-assembly encapsulation or controlled nanoformulation of components, including nanoprecipitation or in situ hydrogel formation, is an increasingly applicable technique for the preparation of this type of functionalized carrier. Self-assembled delivery systems such as micelles, liposomes, and lipid nanoparticles are increasingly interesting due to their biocompatibility, simple preparation, encapsulation of both hydrophilic and hydrophobic cargoes, and some other advantages [83]. The non-covalent fusion or coating of previously prepared nanoparticles with targeting ligands, commonly by electrostatic interactions, is also one of the well-known methods of preparing functional nanoparticles [84].
2. Mannosylated Peptides and Proteins
Mannosylated antigens are one of the most effective ligands for targeting the MR and obtaining high-quality immune responses. Common mannosylated vaccine antigen candidates include peptides and proteins. Chemically explained, glycosylation is used to increase peptide solubility and oral bioavailability to obtain a broader reactivity and to provide more conformational properties to synthetic peptides [85]. Biologically, most molecules involved in the immune system (cellular receptors, cytokines, and antibodies) are glycosylated due to their assembly, stability, cell surface exposure, or secretion and recognition [86]. Additionally, glycosylation increases the specificity of peptide vaccine candidates towards the desired cellular target.
As in nature, synthetic glycosylated proteins and peptides with an MR-tagging ability occur as both O- or N-linked glycol moieties. An earlier study by Gustafson et al. demonstrated MR’s high affinity for both N- and O-linked mannosylated mucin-type fusion proteins [87]. In addition, the mucin-type fusion ovalbumin (OVA) protein carrying multiple oligomannose structures enhances antigen-specific antibody and T lymphocyte responses, thus affecting the humoral and cellular anti-OVA responses in a mice model. This type of protein has great potential to work as a universal antigen-presenting cell (APC)-targeting molecule since it was shown that it can successfully target the carbohydrate recognition domains of MR, MBL, and DC-SIGN receptors [87,88]. Some of our research showed that the mannosylation of previously prepared adamantane-containing desmuramyl peptides resulted in the amplification of its immunostimulating activity in an in vivo mouse model [89,90,91]. Recently, the conjugation of the mannose-based copolymer, synthetic glycol-adjuvant p(Man-TLR7), and OVA resulted in higher humoral and cellular immunity in a mice model when compared to antigens lacking mannose targeting or a TLR7 ligand (Figure 3) [18]. The authors first synthesized p(Man-TLR7), which is a synthetic polymeric glycol-adjuvant composed of two functional monomers that target dendritic cells via mannose-binding receptors or activate DCs via Toll-like receptor 7, using the reversible addition–fragmentation chain transfer (RAFT) polymerization. Then, a copper-free cycloaddition reaction was used to conjugate p(Man-TLR7) to antigens. This method has potentially wide application due to the insensitivity of RAFT polymerization to a wide variety of functional groups and the simplicity of conjugation strategy, which can be used for both amine-containing peptides and whole proteins [92]. Antigen-p (Man-TLR7) conjugates generated a pronounced increase in the magnitude and quality of the humoral immune response, expanding the neutralizing antibody repertoire and antigen-specific memory B cells. Additionally, the newly prepared adjuvant localized immune stimulation to the lymphatic organs, avoiding an acute systemic inflammatory response.
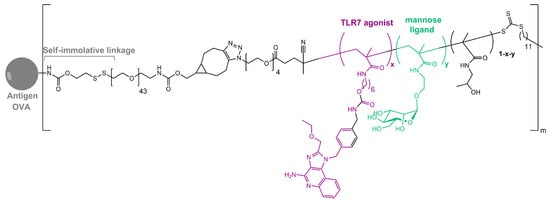
Figure 3. Structure of OVA-p(Man-TLR7).
Human serum albumin (HSA), a non-glycosylated protein, is also an attractive carrier for drug delivery systems [93]. Chemically modified Man-albumins are largely taken up by the liver via an MR-mediated mechanism. Hirata et al. designed and genetically engineered a recombinant oligomannosylated-HSA mutant that can be selectively delivered to the liver via MR on the liver non-parenchymal cells (and, thus, work as a carrier for liver-selective therapeutics) [94]. Recently, a dual-modified albumin in which one molecule of polyethylene glycol (PEG) was conjugated to the Cys34 residue in Man-HSA (Figure 4) was developed. This trivalent system preferably enables the dual-targeting to cancer-associated fibroblast and tumor-associated macrophage-specific MRs (CD206 and CD280), which resulted in a seven times stronger suppression of melanoma growth in B16F10 tumor-bearing mice, when the effect is compared to PEGylated albumin [95]. Furthermore, the PEG-Man-HSA conjugate mediated the disruption of the tumor microenvironment by polarizing tumor macrophages toward the M1 profile.
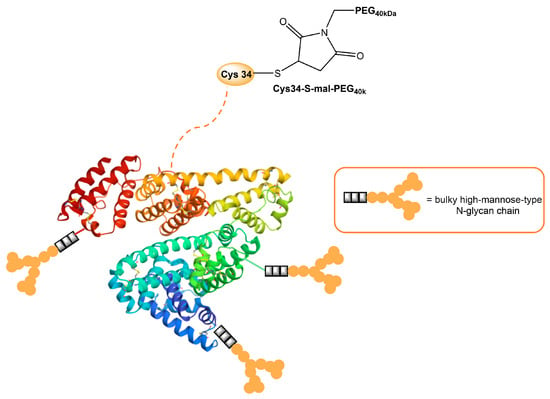
Figure 4. Model of HSA modified with PEG and high-mannose ligands for targeted delivery of paclitaxel to MRs on cancer stoma cells.
A novel tumor-associated mucin MUC1 glycopeptide anticancer vaccine functionalized with covalently linked divalent mannose ligands induced much stronger specific IgG immune responses in mice than the non-mannosylated reference vaccine. Additionally, mannose-binding led to increased numbers of macrophages, dendritic cells, and multiple T cell activation in the local lymph organs. Mannose subunits were introduced using N-terminal mannosyl glycolic acid coupled to both amino groups of a terminal lysine (Figure 5). The tetra-mannosylated conjugate was better recognized by MR and potentially presents a promising target for anti-cancer immunotherapy [15].
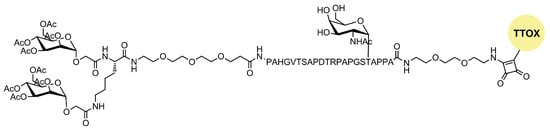
Figure 5. Structure of mannosylated MUC glycopeptide anticancer vaccine.
Over the last couple of decades, peptide-based hydrogels have gained increasing attention for biomedical applications and diagnostic research considering their particular properties, such as their biocompatibility, biodegradability, tuneability,hydrophilic–lipophilic balance of the structure, tissue-like elasticity, and non-cytotoxicity [98]. Considering these properties, recently, Dowari and coworkers prepared a mannose-containing composite hydrogel by combining a self-aggregating short peptide (Nap-FFGE-NH2) and a mannose-containing non-aggregating peptide (Nap-FF-mannosyl) [99]. Nap-FF is a small peptide hydrogelator that can form versatile biofunctional nanofibrous structures [100]. Synthesized composite hydrogel structures provided a potential candidate for the Leishmaniasis treatment via macrophage MR delivery.
Numerous nanotechnologies with advanced functional capabilities were developed [101]. A wide spectrum of these can be used as peptide delivery systems for the improvement of the efficacy of cancer vaccines, which will be discussed later in the paper [102].
3. Mannosylated Lipids and Liposomes
Liposomes are spherical and self-assembled vesicles formed in the aqueous phase from one or more phospholipid bilayers with the polar groups of phospholipid heads oriented to the inner and outer water phases. They are widely used as bioactive platforms for drug and antigen delivery due to their capability to entrap or encapsulate hydrophilic, amphipathic, and lipophilic molecules [103]. Mannosylated liposomes were constantly shown to favorably target macrophages, increasing cellular uptake, both in vitro and in vivo. Mannosylated liposomes can be prepared through the attachment of mannose onto a desired carrier through non-covalent conjugation or by covalently attaching mannose derivatives to liposomes [13]. In the past few decades, numerous strategies have evolved in order to advance a suitable liposome structure for macrophage targeting [9,24,104,105,106]. Several strategies, such as the incorporation of variable amounts and different combinations of phospholipids in the bilayer and the polymeric steric stabilization with hydrophilic PEGs, were attempted to upgrade their in vivo stability [107,108,109]. The incorporation of cholesterol in liposome formulation reduces the permeability of the lipid bilayer, increases liposome stability, and reduces the rapid release of encapsulated bioactive molecules [110]. Spacer length and flexibility between the mannose moiety and the surface of the liposome are also meaningful factors for efficient MR recognition [104,111,112,113]. In addition, the size of the vesicle is important for the circulation half-time of liposomes [103,114].
The in vitro MR-mediated uptake in the APC studies of Sedaghat et al. confirmed the importance of the presence of both mannosyl and lipidic moieties in lipopeptide vaccines for stronger MR binding compared to non-mannosylated compounds [24]. It is well known that the lipidation of peptides is an effective method for improving the metabolic stability and the immune-stimulatory properties of peptide-based vaccines [115,116]. When combined with mannosylation, a specific targeting ability can be achieved. A library of fluorescently labeled mannosylated lipopeptides, with the ovalbumin epitope incorporated (Figure 6), was synthesized using fluorenylmethyloxycarbonyl (Fmoc) solid phase synthesis (SPPS). This research is an extension of previous studies in which it was shown that the distance between the mannose groups plays an important role in the uptake of mannosylated compounds through the MR on monocytes [117,118]. More specifically, shorter spacers between the mannose units were shown to be more effective [9,118]. Recently, Zhang et al. designed mannose-modified liposomes, with antagonistic peptides as co-delivery systems grafted onto a liposome surface, that successfully activated macrophages and stimulated the secretion of various cytokines. The additional hyaluronic acid functionalization of liposomes improved the systemic circulation stability of complexes in vivo and promoted its accumulation in tumor sites [119].
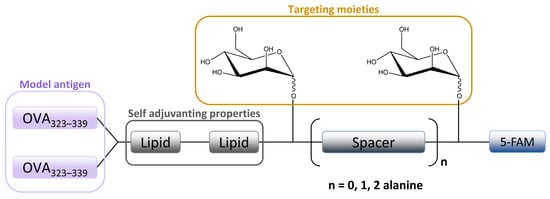
Figure 6. Schematic image of mannosylated lipopeptide vaccines developed by Sedaghat et al. used to target DCs and macrophages MR. Linear arrangement of two mannose units are separated by an alanine spacer attached to the ovalbumine epitope.
Mannose-modified carboxylated curdlan-coated liposomes were prepared and tested for APC selectivity, cross-presentation efficiency, and antitumor effects [120]. Specific in vivo and in vitro tests showed that these liposomes are highly recognized by macrophage cell lines and APC cells in the spleen. Also, liposomes achieved the cytoplasmic delivery of cargo and promoted the cross-presentation of the model antigen, which led to the induction of strong antitumor effects in tumor-bearing mice.
Various mannosylated PEGylated lipids and liposomes were synthesized in order to improve macrophage targeting. Hagimori et al. designed novel, highly functional mannose-PEGylated liposomes with an improved in vitro MR-binding affinity using s serine-glycine repeat spacer between the mannose and lipid moieties [121]. Recently, a nanoprodrug composed of budesonide palmitate and the mannose-PEGylated lipid was prepared. In vitro studies on RAW 264.7 macrophages demonstrated the safety of prodrug administration and anti-inflammatory activity [122]. Different mannose-PEGylated and other polymer-augmented liposomes (PALs) were developed to provide improved cytosolic delivery of streptomycin to alveolar macrophages [123]. Herein, the streptomycin-loaded PALs showed significantly improved intracellular antibacterial activity in a Francisella-macrophage co-culture model when compared with free streptomycin or PEGylated streptomycin liposomes. Also, the mannose targeting capability of the PALs internalization was considerably higher compared to non-targeted PEGylated liposomes. Mannosylated PAL carrier systems designed in this way provide important features for the intracellular delivery of therapeutics with poor membrane permeability. Drug-free mannosylated liposomes and PEGylated liposomes were also recently developed, and cytotoxicity, cellular internalization, immunostimulatory activity, MR-targeting efficiency, and antitumor activity were evaluated in vitro and in vivo [124]. In the obtained results, mannosylated liposomes exhibited superior in vitro cellular internalization and tumor penetration through MR-mediated tumor-associated macrophages (TAMs). Recent studies also based on the design and application of mannosylated liposomes for the purpose of TAM-targeted delivery showed great potential in the regulation of the tumor microenvironment, as well as a promising approach for specific and non-invasive TAM-targeted imaging [125,126,127]. In addition, various PEGylated mannose–cholesterol conjugates were synthesized and used for enhanced in vitro mRNA delivery to DC cells by liposomes [113]. The uptake study revealed that prepared liposomes enhanced mRNA expression mainly through DCs MR, indicating the importance of the MR’s involvement in the mRNA vaccine delivery system.
The codelivery of mannose-modified liposomes, co-encapsulated with dihydroartemisinin and doxorubicin chemotherapeutics, for drug-resistant colon cancer therapy was described by Kang and co-workers [97]. The administration of the Man-liposomes resulted in a tumor inhibition rate of 88.6%, compared to that of 47.5% or 70.5% for the treatment with free doxorubicin or free doxorubicin with dihydroartemisinin, respectively. This combined formulation inhibited the growth of the drug-resistant colon cancer cells and, thus, can be applied for targeted delivery to cancer cells overexpressing the MR.
The comparative binding and uptake of liposomes decorated with various mannose oligosaccharides by cells expressing the MR or DC-SIGN was recently described by Gao and co-workers [128]. A series of glycolipids comprising either mannose, a tri-antenna molecule of α-D-mannopyranoside, the [Manα1-3(Manα1-6)Man], the pseudo-Man4 or pseudo-Man5 were synthesized and embedded in fluorescein-labeled liposomes. A tri-antenna α-D-mannopyranoside X showed the best potential for targeting liposomes to MR (Figure 7).
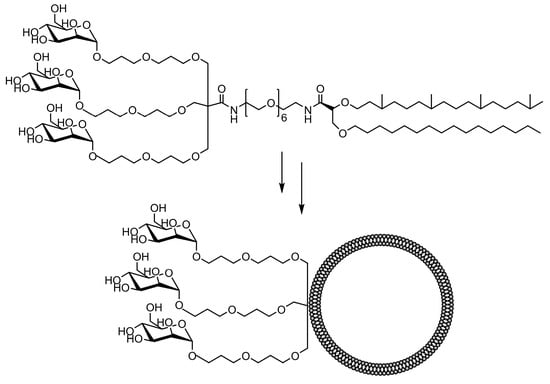
Figure 7. Schematic image of a tri-antenna molecule of α-D-mannopyranoside liposome, tri-Man liposome, prepared by Gao et al [128].
The synthesis and evaluation of numerous other mannosylated liposomal systems for potential MR-macrophage or dendritic cell binding were described elsewhere in the recent literature [129,130,131,132,133,134,135]. Alternatively, oil-in-water (O/W) emulsions were used as colloidal drug carriers for various therapeutic applications and as model particles for cell adhesion modeling [136,137,138]. The functionalized microparticles composed of a fluorescent mannolipid adsorbed on emulsion droplets were used to study the activation of MR-mediated phagocytosis in macrophages [139].
4. Mannosylated Nanoparticles (NPs)
Apart from liposomal systems, numerous other nano-constructs were investigated for their use in effective drug delivery to macrophages, including nanoparticles, carbon nanotubes, dendrimers, micelles, and polymeric particles. Nanoparticles can be prepared in various shapes and sizes, depending on various preparation methods and different starting materials. Different characteristics of nanoparticles, such as size, surface properties, and charge, can influence the interaction between nanoparticles and macrophages. For example, a smaller NP size (<100 nm) is preferred due to the slower removal from blood [140,141]. Moreover, NPs can be designed to specifically stimulate an immune response by interacting with immune components in blood, which causes easier recognition by macrophages [142].
Organic nanoparticles, particularly polymeric nano-objects and micelles, are often mentioned in the recent literature, considering that polymers reduce the often-limiting properties of nanomaterials, such as their low solubility, biocompatibility, and demanding preparation [143,144]. Glycopolymers can be synthesized either by post-polymerization conjugation or by the polymerization of glycosylated monomers. Modern polymerization methods can control the molecular weight, chemical functionality, and polymer architecture [145]. Even though the attachment of mannose onto preformed carriers through non-covalent conjugation includes the involvement of relatively weak nonspecific forces in the interaction between mannose and the carrier surface, which can result in the premature detachment of the ligand before its access to the target, the non-covalent fusion or coating of the previously prepared NPs with targeting ligands is also one of the well-known methods of preparing functional NPs [13,146]. For example, mannose decoration can be achieved via a self-assembly process, in which PEG-poly(propylene oxide)-PEG copolymer (F127-polymer) and tannic acid layers are physically crosslinked with the hydroxyl groups of mannose [147]. As in the case of the preparation of functional liposomes, the conjugation of mannosylated nanoparticles with PEG was used to provide higher stability, solubility, and immunogenicity. In addition, the PEGylation of mannose-modified NPs extended the circulation time and allowed for accumulation in the tumor site in studies related to tumor treatment. For example, Zhu et al. developed PEG-sheddable mannosylated NPs in order to target TAMs in the acidic tumor microenvironment. Recently, PEG-coated calcium zoledronate nanoparticles with conjugate mannose were designed [72]. Zoledronate (Zol) is a third generation of drugs known as bisphosphonates that displayed selective cytotoxicity to TAMs but a short half-life in cancer patients’ circulation [148]. The Zol-NPs specifically targeted TAMs via interaction with macrophage MR, which resulted in enhanced cellular internalization, tumor cell elimination, and restrained tumor growth. In addition, Chen and co-workers studied the optimal structural configuration of mannosylated PEG-conjugate type nanocarriers for targeting the MR on macrophage cells [149]. Their in vitro optimized parameters showed that a small PEG carrier and two mannose units per nanocarrier that are spaced 56 Å apart display the best targeting potential.
Apart from synthetic polymers, the biocompatible and biodegradable lignin polymer recently started to be used as a drug delivery system. Lignin nanoparticles (LNPs) were functionalized with the “mUNO” hexapeptide to target MR on TAMs, which resulted in a meaningful shift in the immune cells in the tumor microenvironment towards an anti-tumor immune state. In addition, the co-administration of the vinblastine with mUNO-LNPs enhanced its antitumor effect [157]. Also, mannosylated biodegradable chitosan nanoparticles were newly developed for macrophage remodeling in case of a fight with a Candida albicans infection. In this paper, mannose motif-enhanced macrophage targeting was emphasized [158]. Various other chitosan-based NPs for drug delivery were recently reported [159,160]. Mannose coating can enhance the uptake of the nanocarriers by alveolar macrophages in the spleen, liver, lymph nodes, and lungs [161,162]. More specifically, mannose significantly increased the uptake of gelatin NPs by alveolar macrophages for Mycobacterium tuberculosis treatment [163]. Gelatin is a natural macromolecule hydrolyzed from collagen, which is highly biocompatible and biodegradable under physiological conditions. When incorporated into mannosylated nano-carrier systems, gelatin is used to create targeted and controllable therapeutic-release carriers for various diseases [164,165,166,167]. Another FDA-approved poly lactic-co-glycolic acid (PLGA) that was a biocompatible and biodegradable polymer was functionalized with mannose, mannan, and mannosamine moieties using a carbodiimide reaction in order to prepare the nanoplatform for macrophage activation in Leishmaniasis therapy [168]. Recently, Zlotnikov et al. designed mannan-grafted cyclodextrin polymers, which showed a great capacity for the loading of antibacterial drugs and their adjuvants, as well as its delivery to macrophage MRs [169]. Within this work, it was presented that oligo- and polymer mannose conjugates grafted with cyclodextrins and polyethyleneimines possess a high loading capacity of 8–20% of the weight of the therapeutic substance and the ability to deliver it to macrophage MRs. In addition, flow cytometry studies showed a high-affinity absorption of the trimannosylated compound on macrophages (95.5%). Other mannose-functionalized NPs self-assembled from cyclodextrins were designed in order to facilitate the delivery of doxorubicin to breast cancer cells that overexpress the MR [170]. Furthermore, Bellato and co-workers pointed out the mannosylation of glycopolycations as an efficient strategy to target immune cells in cancer vaccination via MR [20].
In addition, polymeric micelles are self-assembled amphiphilic polymers with a hydrophobic tail and hydrophilic head where polymer concentrations are above critical micelle concentrations [171]. Depending on the hydrophobic and hydrophilic parts and solvents, micelles can take different shapes, including inverse micelles, spheres, tubules, mixed and cylindrical micelles, worm-like nanocrystal micelles, and so on [172]. In the last few decades, polymeric micelles were extensively used as novel drug vehicles, which can efficiently comprise and deliver various hydrophobic drug molecules to target tissues and subcellular organelles [171,173,174,175,176,177]. Like other nanoparticles, polymeric micelles can be functionalized with specific ligands to improve macrophage targeting efficiency. Recently, mannosylated mixed micelles were developed for the specific delivery of dasatinib (an approved drug for chronic myeloid leukemia) to TAMs, leading to their depletion and the suppression of tumor growth [178]. Also, mannosylated peptide-based micelles were synthesized, and their targeting potential for mannose-binding receptors was studied in order to design a suitable platform for immune modulation [179]. Additionally, mannose-coated polymeric micelles for targeted therapeutic siRNA delivery to human and murine macrophages were successfully synthesized [27]. Mannosylated micelles successfully delivered therapeutic mRNA and induced a shift in gene expression from an M2 phenotype toward an inflammatory M1 phenotype. Yin et al. developed mannosylated polymeric micelles from amphiphilic biodegradable polyesters, further modified with the lipophilic cancer drug doxorubicin. A mannose ligand was introduced via a highly efficient click chemistry protocol, and the authors emphasized that the mannose ligand was responsible for both the stabilization of micelles under physiological conditions and for the active targeting of cancer cells expressing MRs [180].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25031370
This entry is offline, you can click here to edit this entry!
