Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
The emergence of drug resistance combined with limited success in the discovery of newer and effective antimicrobial chemotherapeutics poses a significant challenge to human and animal health. Nanoparticles may be an approach for effective drug development and delivery against infections caused by multi-drug resistant bacteria.
- nanomedicine
- antibacterial efficacy
- drug delivery
1. Introduction
Infectious ailments, especially due to bacteria are a significant burden on public health, killing more than 14 million people annually [1,2]. Molecular components of bacterial pathogens are extensively dissimilar from human cells such as their genetic material, ribosomes, cell membranes, cell wall and biosynthetic routes, hence these aspects are usually exploited to derive antimicrobial agents [3]. In recent years, the development of antimicrobial resistance is proving a major setback in our ability to counter morbidity and mortality associated with bacterial infections [2]. Several strategies have been employed by bacteria to resist antimicrobials including efflux pumps, enzymatic suppression by either hydrolytic degradation or chemical alterations such as the addition of a phosphate group acetylation or hydrolysis, changing target and reprogramming biological synthesis, and accelerated evolution of acquired resistance in microorganisms [4]. The danger of antibacterial resistance is a significant threat to human health worldwide [5,6]. In addition, many antibiotics like fluoroquinolone and aminoglycosides exhibit serious side effects [7]. The majority of multidrug resistance (MDR) infections need extended antibiotic therapy that are accompanied with significant health-care costs [8,9]. Hence, there is a need for the development of new effective antibacterial strategies.
Recently, the applications of nanotechnology especially the use of nanoparticles has been highlighted as a promising solution to the challenges posed by existing antimicrobials. Several nanoparticles have been utilized as delivery vehicles comprising of dendrimers, liposomes, metallic nanoparticles, and polymeric nanoparticles [10]. Size reduction methods and technologies yield different types of nanostructures that exhibit unique physicochemical properties such as magnetism, physical strength, electrical conductance, chemical reactivity and optical effects [11]. These criteria help in increasing the surface area, enhancing release of drug, reducing the dose required, and improving solubility and bioavailability of the compounds [12]. Moreover, these properties are also important for the diagnosis of microbial diseases with high sensitivity and selectivity and those with fluorescent properties or labeled with fluorescent dyes have also been employed for bacterial detection and susceptibility. In addition, inorganic nanoparticle-based diagnostic approaches depend upon the identification of known bacterial genome sequences by directing probes, and thus may not recognize altered and/or novel bacterial strains [13]. Furthermore, nanoparticles based specific drug targeting improve the therapeutic index, increase stability of drug, and decrease drug resistance. For example, silver nanoparticles affect Escherichia coli by forming complex with electron donor groups on amino acids and nucleic acids [12]. At present, liposomal drugs and polymer–drug conjugates have been approved for infectious diseases treatment, and many other antimicrobial nanoparticle formulations are under preclinical test [14].
Gold nanoparticles have gained significant attention recently due to their tunable antimicrobial applications. Gold has been the subject of interest against bacterial infections for its biocompatibility and ease in conjugation with drugs and biomolecules. Gold conjugated with various drugs have shown to enhance their efficacy against bacteria. Gold nanoparticles coated with aminoglycosides have been found to be efficient antibacterial agents against various bacteria such as Staphylococcus aureus, Micrococcus luteus, E. coli and Pseudomonas aeruginosa [15]. In another study, after assessment of the surface chemistry of nanoparticles, the synergistic mechanism showed that hydrophobic cationic conjugated gold nanoparticles reduced the minimum inhibitory concentration (MIC) of fluoroquinolone against multidrug resistant by 8–16 times [16]. More recently, formulations of carbapenem loaded gold nanoparticles with different sizes (35 nm, 70 nm and 200 nm) showed potent antibacterial activity against MDR bacteria including Klebsiella pneumoniae, Proteus mirabilis and Acinetobacter baumanii. Imipenem coated gold nanoparticles showed four-fold decrease in the MIC whereas meropenem decreased the MIC by three-fold [17]. In another report, the antibiofilm activity of gold nanoparticles of around 50 nm and nanoclusters around 2–3 nm stabilized by same ligand, 3-(diphenylphosphino) propionic acid against Staphylococcus aureus and Streptococcus mutans have shown that smaller nanoclusters exhibit better antibacterial effects against gram-positive bacteria [18]. The antibacterial activity of gold nanoparticles depends mainly on their cargo; however, size and shape of gold nanoparticles are also known alter the antibacterial potency. Gold nanoparticles of different shapes (sphere, rod, star and flower) can be prepared by utilizing different chemical protocols, while certain stabilizing and reducing agents can give rise to size selectivity to a very narrow range [19].
2. Therapeutic Efficacy of Nanoparticles
Several aspects can play a role in the therapeutic efficacy of nanoparticles, and this has led to the development of more complex nanoparticles. Many factors play a role in the reaction of nanomaterial with bacteria including hydrophobicity, static electricity attraction, van der Waals forces and receptor–ligand connection which affects the therapeutic potency [20].
In effective targeting, electrostatic interactions occurring between the negative charge of the bacteria surface and the cationic charge of nanoparticles can increase the therapeutic efficacy of nanoparticles [21]. For example, Gold nanorods or nanospheres showed electrostatic interaction with the negative charge of teichoic acid on Bacillus cereus [22]. Mannose substituted gold metal nanoparticles have been shown to bind with the lectin pili as a target on the surface of E. coli [23]. Nanomaterials can interact with intracellular components like respiratory enzymes and DNA to disrupt cellular mechanisms and electrolyte balance, resulting in bacterial lysis [24]. Moreover, the surface chemical composition of nanoparticles is crucial to modify their contact with the bacterial cellular system, improving their therapeutic index while concurrently dropping their toxicity against host cells [11]. For example, Bayraktar (2007) reported that aspartate amino acid functionalized gold nanoparticles bind to large surface of cytochrome c whereas phenylalanine conjugation exhibited much smaller binding surface on cytochrome [25]. In the passive bacterial targeting, the high vascular permeability and impaired function of lymphatic system are resulted in bacterial infection site, which lead to nanoparticles accumulations [26]. For instance, Polyethylene glycerol liposomes favorably located in an intramuscular S. aureus infection site [27].
3. Nanoparticles as Drug Delivery Systems
One of the important uses of nanomedicine is drugs delivery to specific cells and receptors using nanomaterials [60]. Nanoparticles have potential to move through the blood stream, cross the biological barriers effectively and deliver drugs [61]. The transport of drugs to the site of infection is primarily based upon efficient loading of the drugs with the enhanced ability to penetrate cells and overcoming common barriers, as well as response triggered release of the drug [32]. Besides drug loading on nanoparticles, their release also needs to be controlled. A wide diversity of stimuli- reactive polymers have been used to advance nanosystems for drug delivery. These systems show drastic variations in response to various inducements because of creation or disturbing of secondary forces (electrostatic interactions, hydrophobic effects, hydrogen bonding, etc.), solubility, conformation, degradation, bond cleavage and reversibility [62].
There are two main modes for drug release in nano-drug delivery systems, locally chemical stimulated; or externally stimulated. The earlier can occur by simple diffusion and/or through diverse endocytic processes needing chemical and biochemical motivations (i.e., enzymatic activities, hydrolysis, pH, etc.). Otherwise, externally physical activated targeting is centered on external issues, for instance magnet, electrical, ultrasound, temperature and light [62,63]. For example, Gupta et al. (2004) showed that the surface coating of superparamagnetic iron oxide nanoparticles with hydrophilic-hydrophobic polymeric compounds (PEG, poloxamines, poloxamers) was significantly useful for relevant drug delivery by minimizing plasma protein adsorption to the nanoparticles and eliminating reticuloendothelial system uptake [64]. Food and Drug Administration accepted Visudyne, stimuli-responsive nanomedicine, which is consumed for photodynamic therapy [62,65].
As the initial illustration of pH triggered-receptive antibiotic secrete and to release in the specific site, the designation of the nanoparticles relies on acidity gradient of tissues such as skin, digestive tract, and environment inside cells [66]. Chitosan altered gold nanoparticles was connected to the liposomal surface to stop the undisciplined union of spherical vesicles. At low acidic PH rates, the attached gold nanoparticles separate from liposomes and the existence of microbial toxins damaged the liposome bilayer membranes to liberate antibiotics [67]. Ji et al. (2016) demonstrated the bacterial toxin triggered drug delivery [68]. Furthermore, in small molecule conjugated nanoparticles, the charges of both liposome and nanoparticles affect PH stimuli responsive connecting and disconnecting of nanoparticles. For example, liposome- and gold-nanoparticles bind with bacteria at bodily and acidic PH depending on charge distribution [67,69]. For enzyme responsive polymeric nanoparticles, Xiong et al. (2012) used lipase polymeric compound as a drug delivery carrier, once the layers of complex discerned the lipase liberating S. aureus, the layers are degraded to release vancomycin. Graphene-mesoporous silica surrounded by hyaluronic acid with overloaded ferromagnetic nanoparticles and ascorbic acid. When the conjugated compound accesses the infection location, bacteria release the hyaluronidase which in turn destroy hyaluronic acid. This disruption lead to ascorbic acid changing to hydroxyl radicals, followed by membrane devastation of bacteria [70].
4. Nanoparticles Cytotoxicity
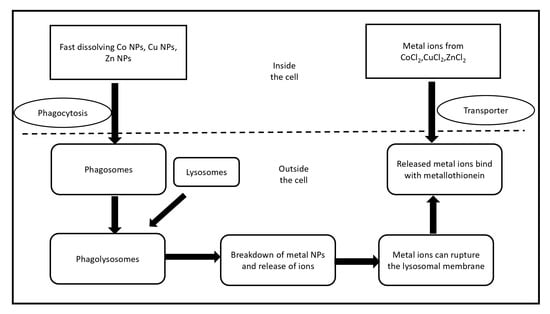
The toxicity of nanoparticles has remained a significant concern, which has limited their clinical applications. Creation of reactive oxygen species is one of chief mechanisms of nanotoxicity influenced by nanoparticle surface chemistry and surface charges [71]. There is no clear distinction among nanomaterials for their cytotoxicity, and there are several contradictory reports based on the design of experiments, types of nanomaterials, choice of cells etc. The capture of nanoparticles by endocytosis and enhancing intracellular death due to faint cell-nanoparticles adhesive interactions are considered acceptable explanation for increasing the cytotoxicity in case of uncoated nanoparticles. The high cytotoxicity in relation to increasing nanoparticles concentration, the surface chemical aspects of nanoparticles determine nanoparticle–cell interaction, adsorption way, and cell behaviour on contact [66]. Isabel et al. (2017) reported different types of metal nanoparticles against viability and morphology of cerebral cells [72]. However, it is important to note that these materials have been tested as drug vectors in other cell lines without affecting viability [73]. In a recent report, the effect of biogenic silver nanoparticles on breast cancer cell line (MCF-7) cells at specific IC50 doses resulted in apoptosis through increased ROS and decrease in membrane potency of mitochondria in addition to cell cycle arrest and DNA shattering causing oxidative burst and mitochondrial function failure in the way of MCF-7 death [74]. Jeong et al. (2018) showed the differential comparing in the cytotoxic effects and mechanism of toxicity of rapid dissolving metallic oxide nanoparticles (CuO, CoO, and ZnO) and their metal ions constituent using related epithelial cells for inhalation environment. The potential cytotoxicity of CoO NPs and CuO NPs showed similarities while as ZnO NPs showed a much less cytotoxicity, because of Zn-metallothionein that can behave as antioxidant, compared to their respective metal chloride [75]. This difference in toxicities may be caused by various intracellular absorption of these materials and their interactions as shown in Figure 1.

Figure 1. Schematic illustration for the different cytotoxic influence of rapid dissolving metal oxide nanoparticles and their specific metal chlorides. Nanoparticles cross the cell by phagocytosis forming phagosomes which fuse with spherical lysosomal organelle to arise phagolysosomes. In the low PH fluid of lysosome, analysis of nanoparticles and its metal ions can break lysosomal layer followed by the resulted free metal ions attach with metallothionein, while the membrane transporters are responsible for entering metal ions, then bind to metallothionein.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics8040260
This entry is offline, you can click here to edit this entry!
