Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Water Resources
地下水是淡水的重要来源,约占地球上可用淡水资源总量的95%。它不仅用于日常用水需求,还用于农业灌溉、工业用途、生态补给和发电。
- high-arsenic groundwater
- worldwide scale
- in situ remediation of arsenic
- human health risk assessment
[1]1. 引言
Groundwater constitutes a vital source of freshwater, accounting for roughly 95% of the total available freshwater resources on Earth [1]. It is utilized not only for daily water needs but also for agricultural irrigation, industrial purposes, ecological recharge, and power generation [2]. Therefore, groundwater holds significant value as a resource and plays a critical role in the environment. The degradation of groundwater quality represents a significant issue within the context of global environmental and climate change today. Since the Industrial Revolution, there has been widespread concern over the deterioration of groundwater quality [3]. Among the various groundwater quality issues, the release of high concentrations of heavy metals has had a significant impact on groundwater quality, and serious consideration must be given to its potential risks and hazards to human health. In particular, As is considered by the United States Agency for Toxic Substances and Disease Registry (ATSDR) to be the pollutant that poses the highest potential risk to human health due to the release from natural sources and the resulting high geogenic concentrations in groundwater [4]. The sources of As in groundwater primarily include natural origins such as geological formations, volcanic activity, and hydrothermal processes, as well as anthropogenic activities including mining, coal combustion, and petroleum extraction [5]. The majority of global health issues caused by As are linked to the consumption of water with high As concentrations. Due to the wide range of negative effects of high As concentrations on human health, the World Health Organization (WHO), the United States, and the European Union (EU) have lowered the Maximum Contaminant Level (MCL) of As in drinking water from 50 μg/L to 10 μg/L as a safe limit for As concentration in drinking water [6,7]. High-As groundwater is defined as groundwater with As concentrations above the WHO drinking water standard. The enrichment of high-As groundwater is primarily influenced by a combination of natural sources and hydrogeochemical conditions, with the majority of natural high-As groundwater primarily being a result of geological arsenic contamination [5]. Despite the established risks, many countries, such as Bangladesh, Nepal, Pakistan, Mexico, and Argentina, continue to adopt the 50 µg/L standard for arsenic concentration in their national drinking water guidelines, due to a lack of professional expertise, economic considerations, and the low-level arsenic detection technology [8].
Human exposure to As occurs through direct and indirect pathways. Direct exposure involves drinking water with a high As concentration, contact with skin, and inhalation of gasses with a high As concentration. Indirect exposure mainly occurs through the food chain; this includes eating crops, vegetables, and fruits cultivated in As-contaminated soil or irrigated with As-rich groundwater, as well as consuming meat products from animals raised in such environments. Prolonged exposure to As, regardless of the route, can result in serious health disorders affecting the skin, blood vessels, and nervous system. Extended periods of high As exposure also notably increase the risk of developing cancers in organs like the lungs, liver, kidneys, and skin [9,10] (Figure 1).
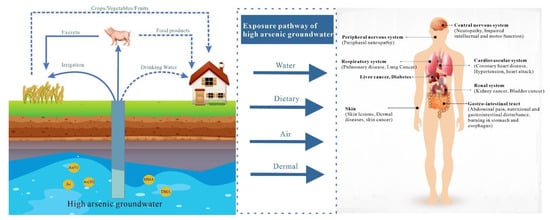
Figure 1. Different pathways of arsenic exposure in groundwater and effects on humans (MMA-Monomethylarsenite; DMA-Dimethylarsenite).
Environmental As exists in groundwater in both organic and inorganic forms, with varying levels of toxicity associated with different forms. The three primary forms of inorganic arsenic are as follows: pentavalent arsenate [As(V)], trivalent arsenite [As(III)], and metallic arsenic. Arsenic in organic form often occurs as various organic arsenic compounds such as Monomethylarsenite (MMA) and Dimethylarsenite (DMA) [11]. Among these, inorganic arsenic is more toxic to humans, and the toxicity significantly differs between the oxidation states of As(III) and As(V). The toxicity of As(III) is more than 60 times higher than that of As(V) and 70 times higher than that of methylated arsenic [12]. The heightened toxicity of As(III) is partially because of its reactivity towards biologically relevant molecules [13]. The methylated arsenic forms, including MMA and DMA, exhibit moderate toxicity, while other organic forms, such as arsenobetaine (AsB) and arsenocholine (AsC), are generally considered non-toxic [12]. In aqueous solutions, As(III) and As(V) primarily exist as oxyanions due to the high charge and small ion radius of As3+ and As5+. The presence and dispersion of distinct arsenic compounds within hydrological systems are markedly influenced by both the redox potential and pH levels prevailing in aquatic environments [14]. Under circumstances characterized by moderate-to-high redox potentials, As tends to stabilize into the As(V) form (H3AsO4, H2AsO4−, HAsO42−, or AsO43−). Conversely, in environments featuring predominantly acidic or weakly alkaline reducing conditions, and lower redox potentials, As(III) tends to be prevalent as the uncharged H3AsO3 molecule [15].
2. Global Distribution of Geogenic High-Arsenic Groundwater
High-As groundwater is widespread worldwide. According to statistics, 107 countries are affected by high-As groundwater, with the highest number in Asia (32) and Europe (31), followed by Africa (20), North America (11), South America (9), and Australia (4) [16]. The most affected countries are Bangladesh, India, Pakistan, China, Nepal [7], Laos [17], Cambodia [18], Myanmar [19], Vietnam [20], and the United States. The world map (Figure 2) displays the global distribution of geogenic high-As groundwater, predominantly found in inland basins and river deltas in South Asian, East Asian, and South American countries. Major countries are shown in Table 1. Generally, the river-marine sedimentary shallow (Holocene) aquifers in the river deltas are the main areas where high-As groundwater occurs naturally, and it occurs mainly under reducing aquifer conditions [21].
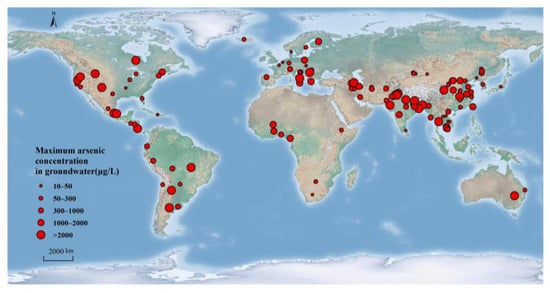
Figure 2. Main countries worldwide affected by geogenic high-arsenic groundwater (≥10 μg/L) (taking the maximum arsenic concentration), see Table 1 for specific data.
Globally, the problem of geogenic high-As groundwater is particularly prominent in South and Southeast Asia, especially in Bangladesh and India [22]. In Bangladesh, 61 areas have been identified as having high-As groundwater. The potential population at risk is approximately 20 million people [23]. According to the National Drinking Water Survey of Bangladesh, around 8% of the water samples had As levels exceeding the Bangladesh standard of 50 μg/L, while around 18% of the samples were above the WHO guideline of 10 μg/L [24]. The concentration of As in groundwater is higher in Bangladesh compared to other countries, and some tube wells even contain As concentrations as high as 4730 μg/L [25,26]. In India, high-As groundwater has already affected twenty states and four union territories, and about 100 million people are under threat from the toxicity of high-As groundwater [16,27,28,29]. The impact of high-As groundwater in India is concentrated on the Ganges–Yarlung Tsangpo Plain, seen on the neo-alluvial (Holocene) floodplains of the rivers in the Himalayas [30,31]. Approximately 50–60 million individuals in Pakistan consume high-As groundwater (>50 μg/L) in vulnerable areas [32]. A meta-analysis of groundwater affected by As in Pakistan showed that 73% of these groundwater samples contained arsenic above 10 µg/L [33]. China is also one of the world’s most representative areas of high-As groundwater, with more than 20 provinces/autonomous regions having high-As groundwater problems. These high-As groundwater provinces are mainly located in the fluvial/alluvial-lacustrine plains and basins (Yinchuan Plain, Hetao Plain, Guide Basin, Hohhot Basin, Junggar Basin, Datong Basin, etc.) located in arid/semi-arid regions and alluvial plains/basins and river deltas in humid/semi-humid regions (Yangtze River, Yellow River Delta, Pearl River Delta, Delta, Huaihe River, Alluvial Plain, Yellow River, Yuncheng Basin, Taiyuan Basin, Songnen Plain, etc.) [34,35,36,37]. The population affected by high-As groundwater contamination in China was estimated to be about 19.6 million according to a statistical risk assessment model developed by Rodríguez-Lado et al. [38].
Table 1. The occurrence of high-As groundwater reported by major countries in the world.
| Country | Study Area | Max As conc. (µg/L) | Samples | Environmental Condition and/or Enrichment Mechanism | References |
|---|---|---|---|---|---|
| Afghanistan | Ghazni and maidan Wardak provinces | 990 | 746 | The weathering and leaching action | [39] |
| Argentina | Santiago del Estero Province | 14,969 | 40 | Volcanic ash sedimentary environment; agricultural irrigation | [40] |
| La Pampa | 5300 | 44 | The geological factors; weathering of volcanic ash and loess; oxidizing condition | [41] | |
| Australia | Stuarts Point coastal | 85 | 140 | Desorption of As from Al-hydroxides and As-enriched Fe-oxyhydroxides; high concentrations of HCO3− and PO4− | [42] |
| Bangladesh | Noakhali | 4730 | 52,202 | Eroded by flood plain rivers | [25] |
| Bolivia | 364 | 24 | The alteration of volcanic rocks; evaporation and redox reactions | [43] | |
| Botswana | Botswana | 116 | 20 | Delta; evaporation concentration; weakly alkaline environment; pH 6.29–8.60 | [44] |
| Brazil | 2980 | Anthropogenic; volcanic activity and weathering of rocks | [43] | ||
| Burkina Faso | 1630 | 45 | Zones of gold mineralization in volcano-sedimentary rocks | [45] | |
| 中国 | Datong Basin | 1932 | 1022 | The weak alkaline reductive environment; high HCO3− concentration; water–rock interactions | [46] |
| Hetao Basin | 572 | 63 | The reducing conditions; the dissolved organic; the competitive effects of other anions | [47] | |
| 江汉盆地 | 2330 | 34 | The high HCO3−浓度;微生物和外源性物质;季节变化; 强烈减少环境; 减少环境 |
[48] | |
| 台湾(兰阳和嘉南平原) | 1010 | 冲积平原;高 DOC;强还原条件 | [49] | ||
| 塔里木盆地 | 91.2 | 233 | 减少环境;溶解的有机物;还原溶出释放; | [50] | |
| 银川 | 177 | 92 | 农业灌溉;Fe氧化物的还原溶解;高PO4−浓度 | [51] | |
| 珠江三角洲 | 161 | 18 | 还原环境;高NH4+浓度;高浓度的NH4+和有机物 | [52] | |
| 柬埔寨人 | 1610 | 207 | 全新世冲积沉积物;减少环境 | [53] | |
| 哥斯达黎加 | 哥斯达黎加北部 | 29,100 | 35 | 与火山岩有关 | [43] |
| 捷克共和国 | 莫克尔斯科 | 1690 | 62 | pH值> 9 | [54] |
| 厄瓜多尔 | 969 | 67 | 泡温泉 | [43] | |
| 埃塞俄比亚 | 埃塞俄比亚西南部 | 184.5 | 44 | pH值< 7 | [55] |
| 加纳 | 1760 | 357 | 地雷泄漏;pH 值 4.8–6.99 | [56] | |
| 匈牙利 | 匈牙利南部 | 260 | 73 | 深度为 0.8–2.4 km,包含 CH4 | [57] |
| 印度 | 海尔 | 1466 | 1365 | 恒河平原;全新世较新的冲积层和更新世较旧的冲积层 | [58] |
| Shahpur 街区,Bhojpur 区,比哈尔邦 | 1805 | 4704 | 恒河平原 | [28] | |
| 旁 遮 普 | 3192 | 4780 | 冲积含水层 | [58] | |
| 伊朗 | 库尔德斯坦 一些村庄 | 1500 | 27 | 采矿和沉积环境 | [59] |
| 东阿塞拜疆-大不里士平原 | 2000 | 18 | 水文地质和 环境还原条件 |
||
| 阿尔达比勒-A市 | 5834 | 163 | 热液流体与岩石和地质源地质构造的相互作用 | ||
| Mazandar an-Haraz 河 | 110 | 20 | 地质来源和采矿 | ||
| 塔巴斯南呼罗珊 | 53 | 29 | 风化 | ||
| 拉扎维·呼罗珊·楚普·克什玛尔 | 606 | 12 | 地质 成因沉积环境 |
||
| 伊斯法罕穆特金矿区 | 1061 | 17 | 风化和采矿 | ||
| 日本 | 38 | 136 | 减少环境和工厂排污 | [26] | |
| 韩国 | 锦山县 | 113 | 150 | 变质沉积岩中硫化物矿物的氧化反应及高pH条件下的解吸过程 | [60] |
| 尼日利亚 | 瓦里-哈科特港,奥贡州,卡杜纳 | 750 | 20 | 冲积沉积物,还原环境,微酸性 | [16] |
| 巴基斯坦 | Kasur、Shhiwal、Bahawalpur 和 Rahim Yar Khan | 3090 | 395 | 灌溉和工厂污水 | [61] |
| 拉合尔市 | 85 | 41 | 表土和非承压含水层的广泛灌溉,还原溶蚀 | [32] | |
| 梅尔西 | 812 | 44 | 人类活动 | [49] | |
| 巴拉圭 | 120 | 37 | 人类活动和火山灰沉积环境 | [43] | |
| 老挝人民民主共和国 | 万象 | 24.4 | 3 | 减少环境 | [17] |
| Borikhamxay(博里坎赛酒店) | 30 | 7 | 减少环境 | ||
| 尚帕萨克 | 25.6 | 27 | 减少环境 | ||
| 阿速坡 | 31.6 | 10 | 减少环境 | ||
| 缅甸 | 伊洛瓦底江 | 630 | 55 | 氢氧化铁的还原溶解 | [49] |
| 墨西哥 | 拉古纳地区 | 5000 | 29 | 在氧化铁、粘土矿物表面和有机碳上的吸附或共沉淀 | |
| 萨卡特卡斯 | 75.4 | 182 | 地质成因, 水-岩相互作用 | [49] | |
| 尼泊尔 | 纳瓦帕拉西 | 2620 | 18,000 | 季节与气候变化,水岩相互作用 | |
| 巴基斯坦 | 拉尔卡纳信德省, | 318 | 58 | pH 值 6.8–8.1 | [62] |
| 旁 遮 普 | 655 | 141 | pH 值 7.0–9.3 | [63] | |
| 西班牙 | 杜罗新生代盆地 | 613 | 514 | pH 值 5.87–1.58 | [64] |
| 泰国 | 素攀武里府 | 5000 | 21 | pH 值 5.20–5.90;EH 250–370 毫伏 | [16] |
| 美国 | 加利福尼亚州圣华金谷 | 148.5 | 4983 | 干旱和半干旱盆地; 冲积、河流和湖泊沉积物;pH>7.8;还原条件 |
[65] |
| 内华达州丘吉尔县的拉洪坦谷 | 4100 | 59 | 湖泊沉积物 | [66] | |
| 越南 | 湄公河三角洲 | 850 | 109 | pH 值 7.22–8.63 | [49] |
在欧洲,地下水的污染归因于地热和热液系统,以基岩和火山沉积物为主[67]。潘诺尼亚盆地(罗马尼亚,塞尔维亚和匈[2]牙利)的情况尤其值得注意,因为超过600,000名居民可能暴露于高砷地下水[57]。此外,芬兰基岩地下水中砷的最大浓度为 1040 μg/L。意大利南部伊斯基亚岛地区记录到的最高砷浓度为1479微克/升,是MCL的148倍。热液活动和热控制似乎是导致砷从矿物中释放的主要因素[68]。
美国和加拿大也经历了广泛的地质成因高砷地下水污染,但其浓度低于亚洲国家[69]。在拉丁美洲,地下水中的砷化合物主要来源于地热流体和火山活动[43]。在墨西哥31个州中,有13个州的饮用水含量过高[70]。特别是,在La Laguna地区的孔隙弱渗透层中发现了5000 μg/L的浓度[71]。地下水:在瓜纳华托州的Juventino Rosas和墨西哥州的Ixtapan de la Sal和Tonatico已经确定了地下水作为地热来源[72]。阿根廷地下水中受As影响最大的地区是Chaco-Pampean平原,在收集的86个地下水样本中,约有88%超过了WHO的指导值,阿根廷的高危人群约为400万人[54]。
This entry is adapted from the peer-reviewed paper 10.3390/w16030478
References
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O.; Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259-284, .
- P.L Smedley; H.B Nicolli; D.M.J Macdonald; A.J Barros; J.O Tullio; Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259-284, .
- Burri, N.M.; Weatherl, R.; Moeck, C.; Schirmer, M. A Review of Threats to Groundwater Quality in the Anthropocene. Sci. Total Environ. 2019, 684, 136–154.
- ATSDR. The ATSDR 2019 Substance Priority List, Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 5 November 2023).
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and Temporal Variations of Groundwater Arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127.
- Gorchev, H.G.; Ozolins, G. WHO Guidelines for Drinking-Water Quality. WHO Chron. 1984, 38, 104–108.
- Thakur, J.K.; Thakur, R.K.; Ramanathan, A.L.; Kumar, M.; Singh, S.K. Arsenic Contamination of Groundwater in Nepal—An Overview. Water 2011, 3, 1–20.
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A Review on Decontamination of Arsenic-Contained Water by Electrocoagulation: Reactor Configurations and Operating Cost along with Removal Mechanisms. Environ. Technol. Innov. 2020, 17, 100519.
- Rahaman, M.S.; Rahaman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental Arsenic Exposure and Its Contribution to Human Diseases, Toxicity Mechanism and Management. Environ. Pollut. 2021, 289, 117940.
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and Management of Arsenic Pollution in Groundwater: A Comprehensive Appraisal of Recent Global Scenario, Human Health Impacts, Sustainable Field-Scale Treatment Technologies. J. Environ. Chem. Eng. 2021, 9, 105203.
- Hung, D.Q.; Nekrassova, O.; Compton, R.G. Analytical Methods for Inorganic Arsenic in Water: A Review. Talanta 2004, 64, 269–277.
- Kumaresan, M.; Riyazuddin, P. Overview of Speciation Chemistry of Arsenic. Curr. Sci. 2001, 80, 837–846.
- Kalman, J.; Smith, B.D.; Bury, N.R.; Rainbow, P.S. Biodynamic Modelling of the Bioaccumulation of Trace Metals (Ag, As and Zn) by an Infaunal Estuarine Invertebrate, the Clam Scrobicularia Plana. Aquat. Toxicol. 2014, 154, 121–130.
- Ansari, M.A.; Saravana Kumar, U.; Noble, J.; Akhtar, N.; Akhtar, M.A.; Deodhar, A. Isotope Hydrology Tools in the Assessment of Arsenic Contamination in Groundwater: An Overview. Chemosphere 2023, 340, 139898.
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235.
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079.
- Cho, K.H.; Sthiannopkao, S.; Pachepsky, Y.A.; Kim, K.-W.; Kim, J.H. Prediction of Contamination Potential of Groundwater Arsenic in Cambodia, Laos, and Thailand Using Artificial Neural Network. Water Res. 2011, 45, 5535–5544.
- Buschmann, J.; Berg, M.; Stengel, C.; Sampson, M.L. Arsenic and Manganese Contamination of Drinking Water Resources in Cambodia: Coincidence of Risk Areas with Low Relief Topography. Environ. Sci. Technol. 2007, 41, 2146–2152.
- Van Geen, A.; Ahmed, E.B.; Pitcher, L.; Mey, J.L.; Ahsan, H.; Graziano, J.H.; Ahmed, K.M. Comparison of Two Blanket Surveys of Arsenic in Tubewells Conducted 12years Apart in a 25km2 Area of Bangladesh. Sci. Total Environ. 2014, 488–489, 484–492.
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Trang, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and Temporal Evolution of Groundwater Arsenic Contamination in the Red River Delta, Vietnam: Interplay of Mobilisation and Retardation Processes. Sci. Total Environ. 2020, 717, 137143.
- Winkel, L.; Berg, M.; Amini, M.; Hug, S.J.; Annette Johnson, C. Predicting Groundwater Arsenic Contamination in Southeast Asia from Surface Parameters. Nat. Geosci. 2008, 1, 536–542.
- Brammer, H.; Ravenscroft, P. Arsenic in Groundwater: A Threat to Sustainable Agriculture in South and South-East Asia. Environ. Int. 2009, 35, 647–654.
- Ganguli, S.; Rifat, M.A.H.; Das, D.; Islam, S.; Islam, M.N. Groundwater Pollution in Bangladesh: A Review. Grassroots J. Nat. Resour. 2021, 04, 115–145.
- Bangladesh Bureau of Statistics. Bangladesh National Drinking Water Quality Survey of 2009; Bangladesh Bureau of Statistics: Dhaka, Bangladesh, 2011.
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Chandra Mukherjee, S.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S.; et al. Status of Groundwater Arsenic Contamination in Bangladesh: A 14-Year Study Report. Water Res. 2010, 44, 5789–5802.
- Tashdedul, H.M.; Reyes, N.J.D.G.; Jeon, M.; Kim, L.-H. Current Status and Technologies for Treating Groundwater Arsenic Pollution in Bangladesh. J. Wetl. Res. 2022, 24, 142–154.
- Chakraborti, D.; Rahman, M.M.; Chatterjee, A.; Das, D.; Das, B.; Nayak, B.; Pal, A.; Chowdhury, U.K.; Ahmed, S.; Biswas, B.K.; et al. Fate of over 480 Million Inhabitants Living in Arsenic and Fluoride Endemic Indian Districts: Magnitude, Health, Socio-Economic Effects and Mitigation Approaches. J. Trace Elem. Med. Biol. 2016, 38, 33–45.
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic Contamination of Groundwater and Its Induced Health Effects in Shahpur Block, Bhojpur District, Bihar State, India: Risk Evaluation. Environ. Sci. Pollut. R. 2016, 23, 9492–9504.
- Bindal, S.; Singh, C.K. Predicting Groundwater Arsenic Contamination: Regions at Risk in Highest Populated State of India. Water Res. 2019, 159, 65–76.
- Chakraborti, D.; Ghorai, S.; Das, B.; Pal, A.; Nayak, B.; Shah, B. Arsenic Exposure through Groundwater to the Rural and Urban Population in the Allahabad-Kanpur Track in the Upper Ganga Plain. J. Environ. Monit. JEM 2009, 11, 1455–1459.
- Mukherjee, A.; Verma, S.; Gupta, S.; Henke, K.R.; Bhattacharya, P. Influence of Tectonics, Sedimentation and Aqueous Flow Cycles on the Origin of Global Groundwater Arsenic: Paradigms from Three Continents. J. Hydrol. 2014, 518, 284–299.
- Podgorski, J.E.; Eqani, S.A.M.A.S.; Khanam, T.; Ullah, R.; Shen, H.; Berg, M. Extensive Arsenic Contamination in High-pH Unconfined Aquifers in the Indus Valley. Sci. Adv. 2017, 3, e1700935.
- Shahid, M. A Meta-Analysis of the Distribution, Sources and Health Risks of Arsenic-Contaminated Groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319.
- Cao, W.G.; Zhang, Z.; Guo, H.M.; Fu, Y.; Gao, Z.P.; Nan, T.; Ren, Y.; Li, Z.Y. Spatial Distribution and Controlling Mechanisms of High Fluoride Groundwater in the Coastal Plain of Bohai Rim, North China. J. Hydrol. 2023, 617, 128952.
- Wen, D.G.; Zhang, F.C.; Zhang, E.Y.; Wang, C.; Han, S.B.; Zheng, Y. Arsenic, Fluoride and Iodine in Groundwater of China. J. Geochem. Explor. 2013, 135, 1–21.
- He, X.D.; Li, P.Y.; Ji, Y.J.; Wang, Y.H.; Su, Z.M.; Elumalai, V. Groundwater Arsenic and Fluoride and Associated Arsenicosis and Fluorosis in China: Occurrence, Distribution and Management. Expo. Health 2020, 12, 355–368.
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A Review of High Arsenic Groundwater in Mainland and Taiwan, China: Distribution, Characteristics and Geochemical Processes. Appl. Geochem. 2014, 41, 196–217.
- Rodríguez-Lado, L.; Sun, G.F.; Berg, M.; Zhang, Q.; Xue, H.B.; Zheng, Q.M.; Johnson, C.A. Groundwater Arsenic Contamination Throughout China. Science 2013, 341, 866–868.
- Saffi, M.H.; Eqrar, M. Arsenic Contamination of Groundwater in Ghazni and Maidan Wardak Provinces: Afghanistan. In Arsenic Research and Global Sustainability: Proceedings of the Sixth International Congress on Arsenic in the Environment (As2016), Stockholm, Sweden, 19–23 June 2016; CRC Press: Boca Raton, FL, USA, 2016; pp. 41–42. ISBN 978-1-138-02941-5.
- Bhattacharya, P.; Claesson, M.; Bundschuh, J.; Sracek, O.; Fagerberg, J.; Jacks, G.; Martin, R.A.; Storniolo, A. del R.; Thir, J.M. Distribution and Mobility of Arsenic in the Río Dulce Alluvial Aquifers in Santiago Del Estero Province, Argentina. Sci. Total Environ. 2006, 358, 97–120.
- Aullón Alcaine, A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.-P.; Mörth, C.-M.; Sracek, O.; Ahmad, A.; Bhattacharya, P. Hydrogeochemical Controls on the Mobility of Arsenic, Fluoride and Other Geogenic Co-Contaminants in the Shallow Aquifers of Northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671.
- Smith, J.V.S.; Jankowski, J.; Sammut, J. Vertical Distribution of As(III) and As(V) in a Coastal Sandy Aquifer: Factors Controlling the Concentration and Speciation of Arsenic in the Stuarts Point Groundwater System, Northern New South Wales, Australia. Appl. Geochem. 2003, 18, 1479–1496.
- Bundschuh, J.; Armienta, M.A.; Morales-Simfors, N.; Alam, M.A.; López, D.L.; Delgado Quezada, V.; Dietrich, S.; Schneider, J.; Tapia, J.; Sracek, O.; et al. Arsenic in Latin America: New Findings on Source, Mobilization and Mobility in Human Environments in 20 Countries Based on Decadal Research 2010-2020. Crit. Rev. Env. Sci. Tec. 2021, 51, 1727–1865.
- Huntsman-Mapila, P.; Mapila, T.; Letshwenyo, M.; Wolski, P.; Hemond, C. Characterization of Arsenic Occurrence in the Water and Sediments of the Okavango Delta, NW Botswana. Appl. Geochem. 2006, 21, 1376–1391.
- Smedley, P.L.; Knudsen, J.; Maiga, D. Arsenic in Groundwater from Mineralised Proterozoic Basement Rocks of Burkina Faso. Appl. Geochem. 2007, 22, 1074–1092.
- He, X.D.; Li, P.Y.; Wu, J.H.; Wei, M.J.; Ren, X.F.; Wang, D. Poor Groundwater Quality and High Potential Health Risks in the Datong Basin, Northern China: Research from Published Data. Environ. Geochem. Health 2021, 43, 791–812.
- Guo, H.; Yang, S.; Tang, X.; Li, Y.; Shen, Z. Groundwater Geochemistry and Its Implications for Arsenic Mobilization in Shallow Aquifers of the Hetao Basin, Inner Mongolia. Sci. Total Environ. 2008, 393, 131–144.
- Wang, Z.; Guo, H.M.; Liu, H.Y.; Zhang, W.M. Source, Migration, Distribution, Toxicological Effects and Remediation Technologies of Arsenic in Groundwater in China. China Geol. 2023, 6, 476–493.
- Wang, Y.X.; Li, J.X.; Ma, T.; Xie, X.J.; Deng, Y.M.; Gan, Y.Q. Genesis of Geogenic Contaminated Groundwater: As, F and I. Crit. Rev. Env. Sci. Tec. 2021, 51, 2895–2933.
- Sun, Y.; Zhou, J.L.; Yang, F.Y.; Ji, Y.Y.; Zeng, Y.Y. Distribution and Co-Enrichment Genesis of Arsenic, Fluorine and Iodine in Groundwater of the Oasis Belt in the Southern Margin of Tarim Basin. Earth Sci. Front. 2022, 29, 99–114.
- Guo, Q.; Guo, H.M.; Yang, Y.C.; Han, S.B.; Zhang, F.C. Hydrogeochemical Contrasts between Low and High Arsenic Groundwater and Its Implications for Arsenic Mobilization in Shallow Aquifers of the Northern Yinchuan Basin, P.R. China. J. Hydrol. 2014, 518, 464–476.
- Wang, Y.; Jiao, J.J.; Cherry, J.A. Occurrence and Geochemical Behavior of Arsenic in a Coastal Aquifer–Aquitard System of the Pearl River Delta, China. Sci. Total Environ. 2012, 427–428, 286–297.
- Berg, M.; Stengel, C.; Trang, P.; Hungviet, P.; Sampson, M.; Leng, M.; Samreth, S.; Fredericks, D. Magnitude of Arsenic Pollution in the Mekong and Red River Deltas—Cambodia and Vietnam. Sci. Total Environ. 2007, 372, 413–425.
- Litter, M.I.; Ingallinella, A.M.; Olmos, V.; Savio, M.; Difeo, G.; Botto, L.; Farfán Torres, E.M.; Taylor, S.; Frangie, S.; Herkovits, J.; et al. Arsenic in Argentina: Occurrence, Human Health, Legislation and Determination. Sci. Total Environ. 2019, 676, 756–766.
- Dilpazeer, F.; Munir, M.; Baloch, M.Y.J.; Shafiq, I.; Iqbal, J.; Saeed, M.; Abbas, M.M.; Shafique, S.; Aziz, K.H.H.; Mustafa, A.; et al. A Comprehensive Review of the Latest Advancements in Controlling Arsenic Contaminants in Groundwater. Water 2023, 15, 478.
- Kusimi, J.M.; Kusimi, B.A. The Hydrochemistry of Water Resources in Selected Mining Communities in Tarkwa. J. Geochem. Explor. 2012, 112, 252–261.
- Rowland, H.A.L.; Omoregie, E.O.; Millot, R.; Jimenez, C.; Mertens, J.; Baciu, C.; Hug, S.J.; Berg, M. Geochemistry and Arsenic Behaviour in Groundwater Resources of the Pannonian Basin (Hungary and Romania). Appl. Geochem. 2011, 26, 1–17.
- Dhillon, A.K. Arsenic Contamination of India’s Groundwater: A Review and Critical Analysis. In Arsenic Water Resources Contamination; Springer: Cham, Switzerland, 2020; pp. 177–205.
- Hamidian, A.H.; Razeghi, N.; Zhang, Y.; Yang, M. Spatial Distribution of Arsenic in Groundwater of Iran, a Review. J. Geochem. Explor. 2019, 201, 88–98.
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and Fluoride Contaminated Groundwaters: A Review of Current Technologies for Contaminants Removal. J. Environ. Manage. 2015, 162, 306–325.
- Tsuji, J.S.; Chang, E.T.; Gentry, P.R.; Clewell, H.J.; Boffetta, P.; Cohen, S.M. Dose-Response for Assessing the Cancer Risk of Inorganic Arsenic in Drinking Water: The Scientific Basis for Use of a Threshold Approach. Crit. Rev. Toxicol. 2019, 49, 36–84.
- Ali, W.; Mushtaq, N.; Javed, T.; Zhang, H.; Ali, K.; Rasool, A.; Farooqi, A. Vertical Mixing with Return Irrigation Water the Cause of Arsenic Enrichment in Groundwater of District Larkana Sindh, Pakistan. Environ. Pollut. 2019, 245, 77–88.
- Mushtaq, N.; Masood, N.; Khattak, J.A.; Hussain, I.; Khan, Q.; Farooqi, A. Health Risk Assessment and Source Identification of Groundwater Arsenic Contamination Using Agglomerative Hierarchical Cluster Analysis in Selected Sites from Upper Eastern Parts of Punjab Province, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 999–1018.
- Gómez, J.J.; Lillo, J.; Sahún, B. Naturally Occurring Arsenic in Groundwater and Identification of the Geochemical Sources in the Duero Cenozoic Basin, Spain. Environ. Geol. 2006, 50, 1151–1170.
- Haugen, E.A.; Jurgens, B.C.; Arroyo-Lopez, J.A.; Bennett, G.L. Groundwater Development Leads to Decreasing Arsenic Concentrations in the San Joaquin Valley, California. Sci. Total Environ. 2021, 771, 145223.
- Walkera, M.; Seiler, R.L.; Meinert, M. Effectiveness of Household Reverse-Osmosis Systems in a Western U.S. Region with High Arsenic in Groundwater. Sci. Total Environ. 2008, 389, 245–252.
- Medunić, G.; Fiket, Ž.; Ivanić, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 183–233. ISBN 9789811385872.
- Daniele, L. Distribution of Arsenic and Other Minor Trace Elements in the Groundwater of Ischia Island (Southern Italy). Environ. Geol. 2004, 46, 96–103.
- Sorg, T.J.; Chen, A.S.C.; Wang, L. Arsenic Species in Drinking Water Wells in the USA with High Arsenic Concentrations. Water Res. 2014, 48, 156–169.
- McClintock, T.R.; Chen, Y.; Bundschuh, J.; Oliver, J.T.; Navoni, J.; Olmos, V.; Lepori, E.V.; Ahsan, H.; Parvez, F. Arsenic Exposure in Latin America: Biomarkers, Risk Assessments and Related Health Effects. Sci. Total Environ. 2012, 429, 76–91.
- Ortega-Guerrero, A. Evaporative Concentration of Arsenic in Groundwater: Health and Environmental Implications, La Laguna Region, Mexico. Environ. Geochem. Health 2017, 39, 987–1003.
- Morales-Arredondo, J.I.; Esteller-Alberich, M.V.; Armienta Hernández, M.A.; Martínez-Florentino, T.A.K. Characterizing the Hydrogeochemistry of Two Low-Temperature Thermal Systems in Central Mexico. J. Geochem. Explor. 2018, 185, 93–104.
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O.; Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259-284, .
This entry is offline, you can click here to edit this entry!
