Dementia is a major cause of poor quality of life, disability, and mortality in old age. According to the geroscience paradigm, the mechanisms that drive the aging process are also involved in the pathogenesis of chronic degenerative diseases, including dementia. The dissection of such mechanisms is therefore instrumental in providing biological targets for interventions and new sources for biomarkers. Within the geroscience paradigm, several biomarkers have been discovered that can be measured in blood and allow early identification of individuals at risk of cognitive impairment. Examples of such markers include inflammatory biomolecules, markers of neuroaxonal damage, extracellular vesicles, and DNA methylation. Furthermore, gait speed, measured at usual and fast pace and as dual task, has shown to detect individuals at risk of future dementia. Here, we provide an overview of available biomarkers that may be used to gauge the risk of cognitive impairment in apparently healthy older adults. Further research should establish which combination of biomarkers possesses the highest predictive accuracy toward incident dementia. Nevertheless, the implementation of currently available markers may allow identification of a large share of at-risk individuals in whom preventive interventions should be implemented to maintain or increase cognitive reserves, thereby reducing the risk of progression to dementia.
- aging
- chronic inflammation
- cognitive frailty
- dual task
- gait
- geroscience
1. Introduction
2. Biological Aging and Its Relationship with Physical and Cognitive Frailty
2.1. Hallmarks of Aging and Their Interaction with Life Course Determinants
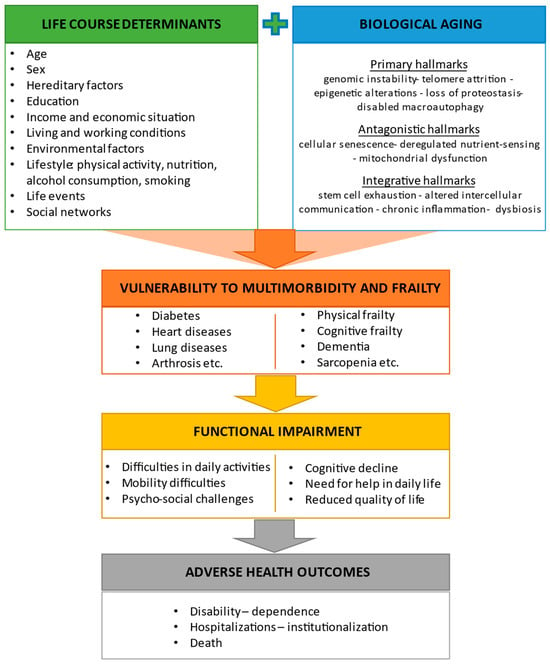
2.2. Frailty, Cognitive Frailty, and Other Predementia Syndromes
3. Biomarkers of Aging Associated with Cognitive Frailty or Cognitive Decline
3.1. Inflammatory Markers
| Biomarker | Study Design and Population | Main Results | Reference |
|---|---|---|---|
| Inflammatory Markers | |||
| CRP | Cross-sectional; individuals ≥60 years (n = 5642) | Higher CRP levels were associated with MCR with memory impairment. | Bai et al. (2021) [48] |
| Panel of inflammatory cytokines and growth factors | Cross-sectional; individuals ≥80 years without severe dementia (n = 415) | IL-6 was associated with cognitive and physical function. | Adriaensen et al. (2014) [49] |
| IL-6 | Cross-sectional; individuals ≥60 years (n = 1340) | Individuals with cognitive frailty had significantly higher serum IL-6 levels compared with controls. | Diniz et al. (2022) [50] |
| CRP, IL-6, TNF-α | Cross-sectional; individuals ≥65 years without dementia (n = 1041) | High CRP and IL-6 serum levels were associated with MCR. | Bortone et al. (2021) [51] |
| Meta-analysis of five cross-sectional studies; older adults (n = 3101) | Circulating IL-6 and CRP were associated with MCR, with associations varying according to the presence of vascular disease. | Groeger et al. (2022) [52] | |
| GDF15 | Cross-sectional; individuals ≥35 years (n = 2736) | Higher plasma GDF15 levels were associated with a combination of cognitive frailty and depression and with cognitive frailty and depressive symptoms separately in younger and older adults. | Kochlik et al. (2023) [53] |
| Progranulin, GDF15, IL-10, IL-6, TNF-α | Cross-sectional; prefrail adults ≥60 years without dementia (n = 397) | Serum TNF-α was significantly elevated in MCR independent of sarcopenia but without obesity. Low IL-10 and IL-10/TNF-α ratio were associated with MCR, independent of sarcopenia and obesity. | Merchant et al. (2023) [54] |
| IL-10 gene polymorphism | Longitudinal (follow-up 3 years); individuals ≥65 years without dementia (n = 530) | Single-nucleotide polymorphisms in the transcriptional regulatory regions of IL-10 gene were associated with incident MCR. | Sathyan et al. (2017) [55] |
| Markers of Neuroaxonal Injury | |||
| NfL | Cross-sectional; individuals ≥45 years with SCD, MCI, or AD (n = 110) | Plasma NfL levels were increased in participants with MCI or AD compared with those with SCD. | Giacomucci et al. (2022) [56] |
| Longitudinal (follow-up 14 years); individuals ≥55 years without dementia (n = 4444) | Higher plasma NfL levels were associated with greater risk of all-cause dementia or AD. Mean NfL concentrations increased 3.4 times faster in participants who developed AD compared with those who remained dementia-free. Plasma values for cases diverged from controls 9.6 years before AD diagnosis. | de Wolf et al. (2020) [57] | |
| Extracellular Vesicles | |||
| Total, neural-, and microglial-derived EVs | Cross-sectional; individuals with and without dementia and frailty, age not reported (n = 60) | Participants with AD had diminished plasma neural EVs levels. Microglial-derived EVs were increased in number in plasma of MCI participants with frailty. | Visconte et al. (2023) [58] |
| MicroRNAs | |||
| Exosomal miRNAs | Longitudinal; four datasets of individuals with and without dementia (n = 544) | A predictive model with six miRNAs (miR29c-5p, miR-143-3p, miR-335-5p, miR-485-5p, miR-138-5p, miR-342-3p) detected preclinical AD 5 to 7 years before the onset of cognitive impairment. | Jia et al. (2022) [59] |
| miRNAs | Longitudinal; two datasets of individuals with and without dementia (n = 147) | The study found that miR-92a-3p, miR-181c-5p, and miR-210-3p were upregulated in plasma of individuals with MCI or AD compared with cognitively healthy participants. Those with MCI who progressed to AD during follow-up showed higher plasma levels of these miRNAs. | Siedlecki-Wullich et al. (2019) [60] |
| miRNA-206 | Longitudinal (follow-up 5 years); individuals with MCI and AD (n = 79) | miRNA-206 was associated with cognitive decline and memory deficits. Changes in plasma levels of miRNA-206 predicted cognitive decline and progression towards dementia in participants with MCI. | Kenny et al. (2019) [61] |
| Longitudinal (follow-up 5 years); individuals with amnestic MCI (n = 458) | During the follow-up, AD was diagnosed in 128/458 participants (28%). Serum levels of miRNA-206 were significantly higher in participants who converted to AD than in those with stable MCI both at baseline and at five years. Serum miRNA-206 was an independent predictor of AD conversion. | Xie et al. (2017) [62] | |
| Epigenetic Clocks | |||
| DunedinPACE | Cross-sectional; individuals ≥55 years with and without dementia (n = 649) Longitudinal (follow-up 14 years; n = 2264) |
DunedinPACE was associated with clinical diagnosis of AD and worse cognitive tests. Participants with more advanced age on the clocks and faster DunedinPACE at baseline were at increased risk of developing dementia during the follow-up. | Sugden et al. (2022) [63] |
| DNA methylation | Longitudinal (follow-up 15 years); individuals ≥55 years with and without dementia (n = 52) | A lower delta age (DNAm age—chronological age) was observed in those with maintained memory functions compared with participants with average or accelerated decline. DNAm age at follow-up, but not chronologic age, was a predictor of dementia. | Degerman et al. (2017) [64] |
| Proteomic Markers | |||
| Plasma proteins | Longitudinal (follow-up 15 years); individuals 20–102 years with and without dementia (n = 997) | Myostatin, peptidase inhibitor 3, trefoil factor 3, and pregnancy-associated plasma protein A were associated with cognitive decline in participants who were cognitively healthy at baseline. | Tanaka et al. (2020) [65] |
| Plasma autoantibodies | Cross-sectional; individuals ≥55 years with and without MCI (n = 236) | Autoantibody biomarkers differentiated participants with MCI from age- and sex-matched controls (accuracy 100%). The autoantibody panel also differentiated those with MCI from participants with mild to moderate AD or other neurologic and non-neurologic diseases. | DeMarshall et al. (2016) [66] |
| Cross-sectional; individuals ≥55 years with and without MCI and dementia (n = 127) | Differential expression analysis identified 33 altered autoantibodies in participants with dementia compared with cognitively healthy controls, and 38 autoantibodies in those with dementia compared with individuals with MCI. | Ehtewish et al. (2023) [67] | |
3.2. Markers of Neurodegeneration
3.3. “Next-Generation” Biomarkers
3.4. Proteomic Biomarkers
4. Frailty and Physical Performance as Predictors of Cognitive Decline
4.1. Frailty
| Biomarker | Study Population and Follow-Up | Frailty, Cognition, and Gait Measures | Main Results | Reference |
|---|---|---|---|---|
| Frailty | ||||
| Physical or cognitive frailty | Cognitively healthy adults ≥65 years (n = 2737); follow-up 4 years | Physical frailty: handgrip strength, BMI, ASM, gait speed, chair-stand test. Cognition: MMSE. |
Most frailty measures at baseline were associated with lower MMSE scores four years later. | Auyeung et al. (2011) [90] |
| Cognitively healthy adults ≥65 years (n = 1045); follow-up 3 years | Physical frailty: Fried’s frailty criteria. Cognition: MoCA. |
Chances of incident cognitive decline were more that twofold greater in individuals with physical frailty than in those with no frailty. | Chen et al. (2018) [86] | |
| Cognitively healthy adults ≥60 years (n = 385); follow-up 7 years | Frailty: Rockwood’s frailty index. Cognition: a neuropsychological test battery. |
Frailty was associated with incident decline in global cognition independent of brain atrophy and cerebral small vessel disease. | Siejka et al. (2022) [87] | |
| Cognitively healthy adults ≥60 years (n = 2150); follow-up 3.5 and 7 years | Reversible cognitive frailty: presence of physical frailty and SCD. Cognition: a cognitive test battery. |
Over a 3.5-year and a 7-year follow-up, participants with reversible cognitive frailty showed an increased risk of incident dementia, particularly vascular dementia. | Solfrizzi et al. (2017) [88] | |
| Cognitively healthy adults ≥65 years (n = 4570); follow-up 3 years | Physical frailty: slow gait speed and muscle weakness. Cognition: a cognitive test battery. |
Cognitive frailty, but not physical frailty without MCI, was a predictor of incident dementia. | Shimada et al. (2018) [89] | |
| Gait Measures | ||||
| Neurologic gait | Cognitively healthy adults ≥75 years (n = 422); follow-up 6.6 years | Gait: neurological gait assessment. Cognition: a neuropsychological test battery and clinical assessment. |
The presence of neurologic gait at baseline was a predictor of dementia, especially vascular dementia. | Verghese et al. (2002) [91] |
| Gait speed | Cognitively healthy adults ≥70 years (n = 1478); follow-up 4 years | Gait: usual gait speed. Cognition: a neuropsychological test battery. |
A faster gait speed at baseline was associated with less cognitive decline. | Mielke et al. (2013) [92] |
| Cognitively healthy adults ≥65 years (n = 660); follow-up 3 years | Gait: usual and fast gait speed, walking-while-talking. Cognition: incident cognitive impairment defined as a ≥3 points loss on MMSE. |
Gait speed at fast pace was associated with cognitive performance at follow-up. | Deshpande et al. (2009) [93] | |
| Cognitively healthy adults ≥60 years (n = 2654); follow-up 6 years | Gait: usual gait speed. Cognition: a cognitive test battery. |
Better performance on executive function, memory, and processing speed was associated with slower decline in gait speed. | Gale et al. (2014) [94] | |
| Cognitively healthy adults ≥75 years (n = 1462); follow-up 7 years | Gait: usual gait speed. Cognition: a cognitive test battery. |
Gait speed was associated with incident dementia independent of body composition parameters. | Van Kan et al. (2012) [95] | |
| Cognitively healthy adults ≥65 years (n = 1042); follow-up 2 years | Gait: usual gait speed. Cognition: 10-word delay recall test. |
A slower baseline gait speed was associated with poorer cognition at follow-up. | Ojagbemi et al. (2015) [96] | |
| Cognitively healthy adults ≥65 years (n = 175); follow-up 14 years | Gait: usual gait speed. Cognition: clinical assessment. |
Gait slowing was associated with cognitive impairment at year 14. A decreased gray matter volume in the right hippocampus on brain MRI was associated with both gait slowing and cognitive impairment. | Rosso et al. (2017) [97] | |
| Individuals ≥65 years with MMSE ≥21(n = 2070); follow-up 7 years | Gait: usual gait speed. Cognition: MMSE. |
Participants with slower gait speed at baseline had a greater rate of cognitive decline at follow-up. | Alfaro-Acha et al. (2007) [98] | |
| Gait speed and variability | Individuals ≥70 with and without dementia (n = 427); follow-up 5 years | Gait: steady state walking using an electronic system. Cognition: a neuropsychological test battery. |
Higher gait variability at baseline was associated with increased risk of incident dementia. | Verghese et al. (2007) [99] |
| Cognitively healthy adults ≥65 years (n = 758); follow-up 2 years | Gait: steady-state walking using an electronic system. Cognition: a cognitive test battery. Frailty: Fried’s frailty criteria. |
Slower gait speed, lower balance confidence, and greater double-support time during walking at baseline were associated with incident cognitive frailty. | Hwang et al. (2023) [100] | |
| Cognitively healthy adults ≥60 years (n = 91); follow-up 4 years | Gait: steady-state walk using triaxial accelerometry-based gait analysis. Cognition: a standardized diagnostic interview. |
Individuals with high gait variability had about a 12-fold greater risk of incident MCI than those with mid to low variability. | Byun et al. (2018) [101] | |
| Dual-task walk | Individuals ≥70 years with MCI (n = 112); follow-up 6 years | Gait: steady-state single- and dual-task walking using an electronic system. Cognition: a neuropsychological test battery. |
A high dual-task walk cost was associated with progression to dementia. | Montero-Odasso et al. (2017) [102] |
| MCR | Individuals ≥65 years with and without cognitive impairment (n = 314); follow-up 1–2 years | Gait: steady-state walking using an electronic system. Cognition: a neuropsychological test battery. |
At baseline, MCR was associated with deficits in attention, language, and overall cognitive status. A slow gait speed and a high gait variability were associated with incident cognitive impairment. | Allali et al. (2016) [103] |
| Cognitively healthy adults ≥60 years (n = 26,802); follow-up 12 years | Gait: steady state walking. Cognition: incident cognitive impairment defined as ≥4 points loss on MMSE. | MCR predicted incident cognitive impairment and dementia. | Verghese et al. (2014) [40] | |
| Cognitively healthy adults ≥60 years (n = 4326); follow-up 2.5 years | Gait: self-reported slow gait. Cognition: incident cases of dementia identified from insurance data. |
MCR was associated with a greater risk of incident dementia. | Doi et al. (2017) [104] | |
| Cognitively healthy adults ≥70 years (n = 997); follow-up 3 years | Gait: steady-state walking using an electronic system. Cognition: a neuropsychological test battery. |
Participants with MCR were at greater risk of developing dementia and vascular dementia. | Verghese et al. (2013) [45] | |
4.2. Gait Speed, Stride-to-Stride Variability, and Gait Speed under Dual-Task Conditions
5. Conclusions
Alterations in circulating levels of several biological markers of aging have been shown to allow identification of individuals at risk of cognitive impairment. For instance, increases in systemic levels of the inflammatory cytokines IL-6 and CRP are associated with MCR, a condition involving a remarkable risk of progression toward dementia. Measurement of plasma levels of NfL, a marker of neuroaxonal damage, may also assist in identifying individuals at their early stages of cognitive deterioration. Likewise, plasma concentrations of myostatin, PI3, TFF3, and PAPPA have shown to recognize at-risk individuals with high accuracy and well before clinical signs of cognitive impairment appear. Third-generation DNA methylation measures of biological aging may also be used to estimate the risk of future cognitive decline. More recently, specific circulating brain derived EVs have been discovered that could be used as predictive biomarkers of neurodegeneration.
Several gait parameters can be used as powerful tools for the prediction of cognitive disorders in clinical practice. A systematic assessment of gait parameters, including a single task walk both at comfortable and fast pace as well as a dual task challenge with simultaneous walking and cognitive tasks, is an inexpensive and readily available method for early detection of individuals at risk of cognitive impairment. The predictive value of gait measurements for cognitive impairment may be enhanced by adding measures of spatiotemporal gait characteristics such as cadence, step width, and stride length, variability of stride time and length, swing time, and double-support time.
Altogether, the available evidence indicates that biological and physical performance markers may enable the identification of individuals at risk of cognitive disorders far earlier than clinical signs or symptoms become detectable. Once a condition of risk is determined, efforts should be made to enhance or maintain cognitive reserves via the elimination or control of modifiable risk factors for dementia. Future studies are needed to establish whether a combination of biological and physical performance markers allow better prediction of incident cognitive impairment than the assessment of either domain alone. Further research is also necessary to evaluate whether changes in biomarkers are sensitive to preventive interventions against dementia and may therefore be used to monitor treatment efficacy.This entry is adapted from the peer-reviewed paper 10.3390/jcm13030806
