Gestational diabetes mellitus (GDM) is a common metabolic disorder that often develops during pregnancy, characterized by glucose intolerance and insulin resistance (IR). To ensure the well-being of both the mother and the fetus, the body undergoes multiple metabolic and immunological changes that result in peripheral IR and, under certain hereditary or acquired abnormalities, GDM in predisposed women. The adverse short- and long-term effects of GDM impact both the mother and the fetus. Nutrition seems to play an important role to prevent GDM or improve its evolution. An emphasis has been given to the proportion of carbohydrates (CHO) relative to protein and lipids, as well as dietary patterns, in GDM. The effects of CHO on postprandial glucose concentrations are reflected in the glycemic index (GI) and glycemic load (GL). Diets rich in GI and GL may induce or exacerbate IR, whereas diets low in GI and GL appear to enhance insulin sensitivity and improve glycemic control. These positive outcomes may be attributed to direct interactions with insulin and glucose homeostasis or indirect effects through improved body composition and weight management.
- gestational diabetes mellitus
- diet
- nutrition
- carbohydrates
- insulin resistance
- glycemic load
- glycemic index
- obesity
- pregnancy
1. Introduction
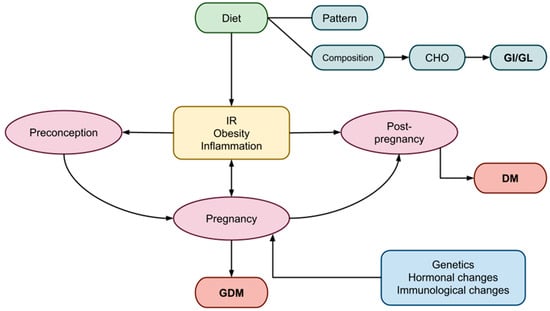
2. Pregnancy
2.1. Gestational IR
2.1.1. Gestational Weight Status
2.1.2. Effects of Diet on Gestational IR
2.1.3. Effects of CHO and GI/GL Estimates on Gestational IR
2.2. Inflammation in Gestation
3. Postpartum Period and Long-Term Management
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/nu16030399
References
- Modzelewski, R.; Stefanowicz-Rutkowska, M.M.; Matuszewski, W.; Bandurska-Stankiewicz, E.M. Gestational Diabetes Mellitus—Recent Literature Review. J. Clin. Med. 2022, 11, 5736.
- Chu, A.H.Y.; Godfrey, K.M. Gestational Diabetes Mellitus and Developmental Programming. Ann. Nutr. Metab. 2020, 76, 4–15.
- Plows, J.; Stanley, J.; Baker, P.; Reynolds, C.; Vickers, M. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342.
- Ladyman, S.R.; Brooks, V.L. Central Actions of Insulin during Pregnancy and Lactation. J. Neuroendocrinol. 2021, 33, e12946.
- Butte, N.F. Carbohydrate and Lipid Metabolism in Pregnancy: Normal Compared with Gestational Diabetes Mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S.
- Duggleby, S.L.; Jackson, A.A. Protein, Amino Acid and Nitrogen Metabolism during Pregnancy: How Might the Mother Meet the Needs of Her Fetus? Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 503–509.
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological Changes in Pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94.
- Rassie, K.L.; Giri, R.; Melder, A.; Joham, A.; Mousa, A.; Teede, H.J. Lactogenic Hormones in Relation to Maternal Metabolic Health in Pregnancy and Postpartum: Protocol for a Systematic Review. BMJ Open 2022, 12, e055257.
- Yahaya, T.O.; Salisu, T.; Abdulrahman, Y.B.; Umar, A.K. Update on the Genetic and Epigenetic Etiology of Gestational Diabetes Mellitus: A Review. Egypt. J. Med. Hum. Genet. 2020, 21, 13.
- Kuang, Y.-S.; Lu, J.-H.; Li, S.-H.; Li, J.-H.; Yuan, M.-Y.; He, J.-R.; Chen, N.-N.; Xiao, W.-Q.; Shen, S.-Y.; Qiu, L.; et al. Connections between the Human Gut Microbiome and Gestational Diabetes Mellitus. Gigascience 2017, 6, gix058.
- Ormazabal, V.; Nair, S.; Carrión, F.; Mcintyre, H.D.; Salomon, C. The Link between Gestational Diabetes and Cardiovascular Diseases: Potential Role of Extracellular Vesicles. Cardiovasc. Diabetol. 2022, 21, 174.
- Nguyen-Ngo, C.; Jayabalan, N.; Salomon, C.; Lappas, M. Molecular Pathways Disrupted by Gestational Diabetes Mellitus. J. Mol. Endocrinol. 2019, 63, R51–R72.
- Abu Samra, N.; Jelinek, H.F.; Alsafar, H.; Asghar, F.; Seoud, M.; Hussein, S.M.; Mubarak, H.M.; Anwar, S.; Memon, M.; Afify, N.; et al. Genomics and Epigenomics of Gestational Diabetes Mellitus: Understanding the Molecular Pathways of the Disease Pathogenesis. Int. J. Mol. Sci. 2022, 23, 3514.
- Dias, S.; Pheiffer, C.; Abrahams, Y.; Rheeder, P.; Adam, S. Molecular Biomarkers for Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 2926.
- Dłuski, D.F.; Wolińska, E.; Skrzypczak, M. Epigenetic Changes in Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 7649.
- Markovic, T.P.; Muirhead, R.; Overs, S.; Ross, G.P.; Louie, J.C.Y.; Kizirian, N.; Denyer, G.; Petocz, P.; Hyett, J.; Brand-Miller, J.C. Randomized Controlled Trial Investigating the Effects of a Low–Glycemic Index Diet on Pregnancy Outcomes in Women at High Risk of Gestational Diabetes Mellitus: The GI Baby 3 Study. Diabetes Care 2016, 39, 31–38.
- Tieu, J.; Shepherd, E.; Middleton, P.; Crowther, C.A. Dietary Advice Interventions in Pregnancy for Preventing Gestational Diabetes Mellitus. Cochrane Database Syst. Rev. 2017, 1, CD006674.
- Damm, P.; Houshmand-Oeregaard, A.; Kelstrup, L.; Lauenborg, J.; Mathiesen, E.R.; Clausen, T.D. Gestational Diabetes Mellitus and Long-Term Consequences for Mother and Offspring: A View from Denmark. Diabetologia 2016, 59, 1396–1399.
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S254–S266.
- Hodson, K.; Dalla Man, C.; Smith, F.; Thelwall, P.; Cobelli, C.; Robson, S.; Taylor, R. Mechanism of Insulin Resistance in Normal Pregnancy. Horm. Metab. Res. 2013, 45, 567–571.
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156.
- Catalano, P.M. Trying to Understand Gestational Diabetes. Diabet. Med. 2014, 31, 273–281.
- Jang, E.-H.; Kwon, H.-S. β-Cell Dysfunction and Insulin Resistance in Gestational Glucose Intolerance. Korean J. Intern. Med. 2013, 28, 294.
- Moreno-Castilla, C.; Hernandez, M.; Bergua, M.; Alvarez, M.C.; Arce, M.A.; Rodriguez, K.; Martinez-Alonso, M.; Iglesias, M.; Mateu, M.; Santos, M.D.; et al. Low-Carbohydrate Diet for the Treatment of Gestational Diabetes Mellitus. Diabetes Care 2013, 36, 2233–2238.
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diab. Rep. 2016, 16, 7.
- Walsh, J.M.; Mahony, R.M.; Culliton, M.; Foley, M.E.; McAuliffe, F.M. Impact of a Low Glycemic Index Diet in Pregnancy on Markers of Maternal and Fetal Metabolism and Inflammation. Reprod. Sci. 2014, 21, 1378–1381.
- Tang, J.; Chen, R.; Yu, Y.; Bao, W.; Tiemeier, H.; Rodney, A.; Zhu, X.; Li, M.; Huang, D.; Zhao, Q. Associations of Pre-Pregnancy Impaired Fasting Glucose and Body Mass Index among Pregnant Women without Pre-Existing Diabetes with Offspring Being Large for Gestational Age and Preterm Birth: A Cohort Study in China. BMJ Open Diabetes Res. Care 2021, 9, e001641.
- Shinar, S.; Berger, H.; De Souza, L.R.; Ray, J.G. Difference in Visceral Adipose Tissue in Pregnancy and Postpartum and Related Changes in Maternal Insulin Resistance. J. Ultrasound Med. 2019, 38, 667–673.
- Gallagher, K.; Migliaccio, L.; Rogers, R.G.; Leeman, L.; Hervey, E.; Qualls, C. Impact of Nulliparous Women’s Body Mass Index or Excessive Weight Gain in Pregnancy on Genital Tract Trauma at Birth. J. Midwifery Womens. Health 2014, 59, 54–59.
- Walsh, J.M.; Byrne, J.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Leptin, Fetal Growth and Insulin Resistance in Non-Diabetic Pregnancies. Early Hum. Dev. 2014, 90, 271–274.
- Powe, C.E.; Huston Presley, L.P.; Locascio, J.J.; Catalano, P.M. Augmented Insulin Secretory Response in Early Pregnancy. Diabetologia 2019, 62, 1445–1452.
- Peppa, M.; Koliaki, C.; Papaefstathiou, A.; Garoflos, E.; Katsilambros, N.; Raptis, S.A.; Hadjidakis, D.I.; Dimitriadis, G.D. Body Composition Determinants of Metabolic Phenotypes of Obesity in Nonobese and Obese Postmenopausal Women. Obesity 2013, 21, 1807–1814.
- Peppa, M.; Koliaki, C.; Hadjidakis, D.I.; Garoflos, E.; Papaefstathiou, A.; Katsilambros, N.; Raptis, S.A.; Dimitriadis, G.D. Regional Fat Distribution and Cardiometabolic Risk in Healthy Postmenopausal Women. Eur. J. Intern. Med. 2013, 24, 824–831.
- Kizirian, N.V.; Goletzke, J.; Brodie, S.; Atkinson, F.S.; Markovic, T.P.; Ross, G.P.; Buyken, A.; Brand-Miller, J.P. Lower Glycemic Load Meals Reduce Diurnal Glycemic Oscillations in Women with Risk Factors for Gestational Diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000351.
- Kunath, J.; Günther, J.; Rauh, K.; Hoffmann, J.; Stecher, L.; Rosenfeld, E.; Kick, L.; Ulm, K.; Hauner, H. Effects of a Lifestyle Intervention during Pregnancy to Prevent Excessive Gestational Weight Gain in Routine Care—The Cluster-Randomised GeliS Trial. BMC Med. 2019, 17, 5.
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519.
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27.
- Louie, J.C.Y.; Brand-Miller, J.C.; Markovic, T.P.; Ross, G.P.; Moses, R.G. Glycemic Index and Pregnancy: A Systematic Literature Review. J. Nutr. Metab. 2010, 2010, 282464.
- Verboven, M.; Deluyker, D.; Ferferieva, V.; Lambrichts, I.; Hansen, D.; Eijnde, B.O.; Bito, V. Western Diet given to Healthy Rats Mimics the Human Phenotype of Diabetic Cardiomyopathy. J. Nutr. Biochem. 2018, 61, 140–146.
- He, L. Alterations of Gut Microbiota by Overnutrition Impact Gluconeogenic Gene Expression and Insulin Signaling. Int. J. Mol. Sci. 2021, 22, 2121.
- Osorio-Yáñez, C.; Gelaye, B.; Qiu, C.; Bao, W.; Cardenas, A.; Enquobahrie, D.A.; Williams, M.A. Maternal Intake of Fried Foods and Risk of Gestational Diabetes Mellitus. Ann. Epidemiol. 2017, 27, 384–390.e1.
- Mustad, V.A.; Huynh, D.T.T.; López-Pedrosa, J.M.; Campoy, C.; Rueda, R. The Role of Dietary Carbohydrates in Gestational Diabetes. Nutrients 2020, 12, 385.
- Schenk, S.; Andrey, M.; De Giorgi, S.; Le Dizes, O.; Puder, J.J. What Is the Place of a Low Carbohydrate or Low Glycemic Index Diet in Gestational Diabetes Treatment? Rev. Med. Suisse 2021, 17, 1083–1086.
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021, 64, 2609–2652.
- Roskjær, A.B.; Andersen, J.R.; Ronneby, H.; Damm, P.; Mathiesen, E.R. Dietary Advices on Carbohydrate Intake for Pregnant Women with Type 1 Diabetes. J. Matern. Neonatal Med. 2015, 28, 229–233.
- Bao, W.; Li, S.; Chavarro, J.E.; Tobias, D.K.; Zhu, Y.; Hu, F.B.; Zhang, C. Low Carbohydrate–Diet Scores and Long-Term Risk of Type 2 Diabetes Among Women With a History of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2016, 39, 43–49.
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy Low-Carbohydrate Dietary Pattern and Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Am. J. Clin. Nutr. 2014, 99, 1378–1384.
- Zhang, X.; Gong, Y.; Della Corte, K.; Yu, D.; Xue, H.; Shan, S.; Tian, G.; Liang, Y.; Zhang, J.; He, F.; et al. Relevance of Dietary Glycemic Index, Glycemic Load and Fiber Intake before and during Pregnancy for the Risk of Gestational Diabetes Mellitus and Maternal Glucose Homeostasis. Clin. Nutr. 2021, 40, 2791–2799.
- Wang, J.-S.; Liu, W.-J.; Lee, C.-L. Associations of Adherence to the DASH Diet and the Mediterranean Diet With All-Cause Mortality in Subjects With Various Glucose Regulation States. Front. Nutr. 2022, 9, 828792.
- Gołąbek, K.; Regulska-Ilow, B. Dietary Support in Insulin Resistance: An Overview of Current Scientific Reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585.
- Critselis, E.; Kontogianni, M.D.; Georgousopoulou, E.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B. Comparison of the Mediterranean Diet and the Dietary Approach Stop Hypertension in Reducing the Risk of 10-Year Fatal and Non-Fatal CVD Events in Healthy Adults: The ATTICA Study (2002–2012). Public Health Nutr. 2021, 24, 2746–2757.
- Mahajan, A.; Donovan, L.E.; Vallee, R.; Yamamoto, J.M. Evidenced-Based Nutrition for Gestational Diabetes Mellitus. Curr. Diab. Rep. 2019, 19, 94.
- Wei, J.; Heng, W.; Gao, J. Effects of Low Glycemic Index Diets on Gestational Diabetes Mellitus. Medicine 2016, 95, e3792.
- Skilton, M.R.; Siitonen, N.; Würtz, P.; Viikari, J.S.A.; Juonala, M.; Seppälä, I.; Laitinen, T.; Lehtimäki, T.; Taittonen, L.; Kähönen, M.; et al. High Birth Weight Is Associated With Obesity and Increased Carotid Wall Thickness in Young Adults. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1064–1068.
- Danielsen, I.; Granström, C.; Haldorsson, T.; Rytter, D.; Hammer Bech, B.; Henriksen, T.B.; Vaag, A.A.; Olsen, S.F. Dietary Glycemic Index during Pregnancy Is Associated with Biomarkers of the Metabolic Syndrome in Offspring at Age 20 Years. PLoS ONE 2013, 8, e64887.
- Hasbullah, F.Y.; Mohd Yusof, B.N.; Shariff, Z.M.; Rejali, Z.; Yong, H.Y.; Mitri, J. Factors Associated with Dietary Glycemic Index and Glycemic Load in Pregnant Women and Risk for Gestational Diabetes Mellitus. Int. J. Food Sci. Nutr. 2020, 71, 516–524.
- Aminianfar, A.; Soltani, S.; Hajianfar, H.; Azadbakht, L.; Shahshahan, Z.; Esmaillzadeh, A. The Association between Dietary Glycemic Index and Load and Risk of Gestational Diabetes Mellitus: A Prospective Study. Diabetes Res. Clin. Pract. 2020, 170, 108469.
- Ojo, O.; Ojo, O.O.; Wang, X.-H.; Adegboye, A.R.A. The Effects of a Low GI Diet on Cardiometabolic and Inflammatory Parameters in Patients with Type 2 and Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1584.
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta 2015, 36, 709–715.
- Szlapinski, S.K.; Hill, D.J. Metabolic Adaptations to Pregnancy in Healthy and Gestational Diabetic Pregnancies: The Pancreas—Placenta Axis. Curr. Vasc. Pharmacol. 2020, 19, 141–153.
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B.; Mosiewicz, J. Associations between Fatty Acid-Binding Protein 4–A Proinflammatory Adipokine and Insulin Resistance, Gestational and Type 2 Diabetes Mellitus. Cells 2019, 8, 227.
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity Increases the Production of Proinflammatory Mediators from Adipose Tissue T Cells and Compromises TCR Repertoire Diversity: Implications for Systemic Inflammation and Insulin Resistance. J. Immunol. 2010, 185, 1836–1845.
- Rehman, K.; Akash, M.S.H. Mechanisms of Inflammatory Responses and Development of Insulin Resistance: How Are They Interlinked? J. Biomed. Sci. 2016, 23, 87.
- Fernández-González, E.; Martínez-González, M.Á.; Bes-Rastrollo, M.; Suescun-Elizalde, D.; Basterra-Gortari, F.J.; Santiago, S.; Gea, A. Association between Pre-Conceptional Carbohydrate Quality Index and the Incidence of Gestational Diabetes: The SUN Cohort Study. Br. J. Nutr. 2023, 129, 704–714.
- Messika, A.; Toledano, Y.; Hadar, E.; Shmuel, E.; Tauman, R.; Shamir, R.; Froy, O. Relationship among Chrononutrition, Sleep, and Glycemic Control in Women with Gestational Diabetes Mellitus: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. MFM 2022, 4, 100660.
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. N. Am. 2016, 100, 1199–1215.
- Egan, A.M.; Dunne, F.P. Optimal Management of Gestational Diabetes. Br. Med. Bull. 2019, 131, 97–108.
- Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S200–S210.
- NICE Guideline Diabetes in Pregnancy: Management from Preconception to the Postnatal. Available online: www.nice.org.uk/guidance/ng3 (accessed on 25 February 2023).
- Tettamanzi, F.; Bagnardi, V.; Louca, P.; Nogal, A.; Monti, G.S.; Mambrini, S.P.; Lucchetti, E.; Maestrini, S.; Mazza, S.; Rodriguez-Mateos, A.; et al. A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study. Nutrients 2021, 13, 4380.
- Looman, M.; Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Geelen, A.; Feskens, E.J.M.; Mishra, G.D. Pre-Pregnancy Dietary Carbohydrate Quantity and Quality, and Risk of Developing Gestational Diabetes: The Australian Longitudinal Study on Women’s Health. Br. J. Nutr. 2018, 120, 435–444.
