Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
The neurobiology of tumors has attracted considerable interest from clinicians and scientists and has become a multidisciplinary area of research. Neural components not only interact with tumor cells but also influence other elements within the TME, such as immune cells and vascular components, forming a polygonal relationship to synergistically facilitate tumor growth and progression.
- head and neck cancer
- nerve–cancer crosstalk
- TME
- tumor neurobiology
1. Introduction
1.1. Nervous System (CNS/PNS)
The nervous system, consisting of the central nervous system (CNS) and peripheral nervous system (PNS), controls a wide range of physiological activities in life, such as organogenesis and development, homeostasis, and regeneration, and modulates many pathophysiological processes, including cancer [1,2]. The CNS, which consists of the brain, brainstem, cerebellum, and spinal cord, regulates tumorigenesis and growth by releasing neurotransmitters or hormones into the systemic circulation [1,3]. In addition, the CNS also functions through the PNS, which acts as a bridge to establish connections between the CNS and the local tumor tissue. The PNS, composed of motor, sensory, and autonomic (sympathetic and parasympathetic) nerve fibers, branches throughout the body, usually accompanied by the microvasculature, infiltrating the tumor microenvironment to modulate vascular or cellular elements [1]. A growing body of experimental and clinical evidence indicates that sympathetic and parasympathetic nerves modulate tumor progression in an antagonistic manner.
1.2. Tumor Microenvironment
The tumor microenvironment (TME) is an ecosystem composed of various cell types, such as endothelial cells, fibroblasts, immune cells, and extracellular matrix components [16,17]. The nervous system has emerged as a new pathological component of the TME and, together with other cellular and non-cellular components, forms a neural–immune–vascular network that plays an important role in tumor initiation, progression, and metastasis. The complex crosstalk between tumor cells and the surrounding environment has been widely recognized to influence therapeutic efficacy [18,19].
The diameters and densities of nerves and their distances from cancer cells are critical features for describing the neurophenotype within the TME and have a clinically relevant impact on the nerve–tumor crosstalk [20]. The nerve diameter is the most fundamental element, and several studies have reported that larger nerve diameters are associated with poor clinical outcomes in several cancer types, including pancreatic, gastric, and head and neck cancers [21,22,23]. The nerve density, defined as the number of nerves in a given area and mainly influenced by axonogenesis and neurogenesis, is another significant predictor of an unfavorable prognosis [24,25,26]. The distance between nerves and cancer cells in the TME, which varies from distant to close but without physical contact to perineural invasion (PNI), is another critical parameter related to tumor metastasis and the death rate [23,27].
1.3. Neurobiology of Head and Neck Tumors
Head and neck cancer, which mainly includes malignancies of the oral and maxillofacial regions and upper aerodigestive tract, is the seventh most common cancer type worldwide, with high morbidity and mortality rates [31,32]. Tobacco use, alcohol consumption, human papillomavirus (HPV) infection, and Epstein–Barr virus (EBV) infection are widely recognized risk factors for head and neck cancer [32,33], and approximately 90% are diagnosed as squamous cell carcinoma (HNSCC) [34]. Traditional treatment options include surgery, chemotherapy, and radiotherapy as monotherapy or in combination, depending on the tumor location and staging. In addition, risk factors such as the resection margin, extracapsular spread, and HPV status also influence postoperative treatment strategies [32,35]. Emerging, new treatment strategies, such as immunotherapy and targeted therapy, demonstrate the increasing focus on key elements of the TME [32,36]. These elements of the TME include neural components, which are common because head and neck cancers, especially oral cancer, exhibit perineural invasion and intra-tumoral innervation to a greater extent than other cancers due to the highly innervated nature of the head and neck region. Recent review articles have summarized biomarkers and neural markers involved in perineural invasion, axonogenesis, and neural reprogramming (discussed in the following chapters), providing compelling evidence for nerve–tumor crosstalk in head and neck cancer [37,38].
3. Perineural Invasion
3.1. Definition, Diagnostics, and Clinical Relevance
PNI is the extension of malignant tumor cells around, into, or through the nerves and was first reported in head and neck cancer as the tendency to spread along the nerves when migrating into the intracranial fossa [40,44]. The predominant pathological definition of PNI is that the tumor is close to a nerve and involves at least 33% of its circumference, or that tumor cells are found in any of the three layers (the epineurium, perineurium, and endoneurium) of the nerve sheaths [40]. The presence of PNI is often a harbinger of tumor-associated pain and has been correlated with a poor prognosis in several cancers, such as colorectal cancer, prostate cancer, and pancreatic adenocarcinoma [45,46,47]. In HNSCC, particularly OSCC, pretreatment pain is also an important variable that is uniquely associated with PNI [48,49]. In HNSCC, the incidence of PNI varies from 25% to 80% [50,51,52], and several studies have positively related PNI to aggressiveness and decreased survival [23,27,53].
Adenoid cystic carcinoma (ACC), which accounts for 1% of head and neck cancers and 7.5–10% of salivary malignancies [54], is characterized by PNI, which provides a low-resistance pathway for metastasis and high invasiveness and leads to pain or nerve paralysis at an early stage [55,56]. However, the effect of PNI on ACC remains controversial, with some studies identifying it as a key prognostic factor or independent predictor of local recurrence and metastasis [57,58,59,60], while other studies show no statistical significance for survival [61,62,63].
3.2. Mode of Mutual Interaction—Schwann Cells
Schwann cells (SCs) are the major supporting glial cells of the PNS that sheathe the peripheral nerve axons and perform several important functions, including rapid signal transduction, nerve trophic support, extracellular matrix production, neurogenesis, and nerve regeneration [64,65]. SCs can partially dedifferentiate into demyelinated repair SCs when nerves are injured or are invaded by tumor cells [66]. Repair SCs produce neurotrophic molecules and secrete pro-inflammatory factors to remodel the local microenvironment and recruit macrophages to synergistically aid axonogenesis and post-injury repair [66,67]. SCs play a key role in promoting PNI. Deborde et al. demonstrated that SCs enhance cancer invasion through direct interaction with tumor cells [68]. SCs express neural cell adhesion molecule 1 (NCAM1), which separates tumor cell clusters into individual cells to induce their migration towards SCs and spread along nerves. In pancreatic and colorectal cancers, SCs migrate to tumor cells, but not to benign cells, via the NGF-neurotrophic receptor tyrosine kinase 1 (NTRK1/TrkA)–nerve growth factor receptor (NGFR/p75NTR) axis before tumor cells initiate migration towards the peripheral nerves, likely providing a pathway for tumor cell invasion [67,69].
3.3. Limitations and Challenges of PNI Diagnostics
Despite the fact that PNI has a critical impact on tumor prognosis, the accurate diagnosis of PNI is still limited, partly due to the lack of standard diagnostic criteria and the intra- and inter-observer variability. In addition, the limited availability of tumor tissue samples from patients undergoing nonsurgical treatment hinders adequate pathological examination. Therefore, studies have focused on deep learning techniques combined with artificial intelligence and bioinformatic analysis to facilitate PNI diagnosis.
4. Intra-Tumoral Neural Infiltration
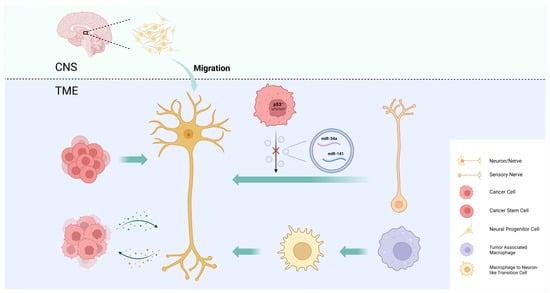
Intra-tumoral nerve infiltration is widely observed in many types of cancer, especially those of highly innervated organs, such as pancreatic cancer, prostate cancer, and head and neck cancer, and it can cause pain, paresthesia, numbness, and paralysis. There is increasing evidence that the intra-tumoral nerve density is associated with tumor progression, metastasis, and prognosis [1,15]. The mechanisms by which tumors regulate intra-tumoral neural infiltration are summarized as axonogenesis, neurogenesis, neural reprogramming, and the transdifferentiation of other cells into neurons (Figure 2).

Figure 2. Mechanisms of intra-tumoral neural infiltration. Tumors regulate intra-tumoral neural infiltration through axonogenesis, neurogenesis, neural reprogramming, and the transdifferentiation of other cells into neurons.
4.1. Axonogenesis
Axonogenesis describes neurite sprouting and outgrowth to provide peripheral nerves to the malignant tumor [15]. Ayala et al. demonstrated axonogenesis in human tumors using two- and three-dimensional reconstructions of whole prostates. The study also found that cancer cells induce neurite outgrowth and axon hyperplasia by secreting the axon guidance molecule semaphorin 4F (SEMA4F), and that silencing SEMA4F inhibits experimental neurogenesis [74]. The study provides new evidence that PNI is not the only interaction between tumor cells and nerves.
4.2. Neurogenesis
In addition to axonogenesis, Ayala et al. were the first to demonstrate cancer-associated neurogenesis in prostate cancer. While axonogenesis refers to neuron enlargement and axon extension, neurogenesis emphasizes the increase in neuron body cells. The study showed an increase in dorsal root ganglion neurons in prostate cancer patients, suggesting neurogenesis [74]. This phenomenon may also be present in other types of cancers, particularly neurotropic cancers that readily invade in, around, and through peripheral nerves, such as pancreatic and head and neck cancers [79].
4.3. Neural Reprogramming
To explore the origin of newly formed adrenergic nerves in the TME of head and neck cancer, Amit et al. compared the transcriptome of tumor-associated trigeminal sensory neurons with that of endogenous neurons and revealed a phenotypic switch from sensory nerves to adrenergic neo-neurons induced by cancer-derived extracellular vesicles (EVs) [39]. This phenomenon has been linked to TP53, one of the most frequently mutated tumor suppressor genes in head and neck cancer, and its fluctuating expression is also closely associated with nerve regeneration [90,91]. In TP53-deficient tumors, a miRNA array analysis revealed a decrease in miR-34a and miR-141 in EVs, which caused an increase in the number of neurofilaments and promoted the axonogenesis of trigeminal ganglion neurons. The reduction in miR-34a also induced the transdifferentiation of sensory nerves towards adrenergic nerves [39]. By performing surgical sensory denervation and chemical sympathectomy prior to tumor cell inoculation in a mouse model, they showed that exosome-induced neural reprogramming, rather than the outgrowth of existing adrenergic nerves, promoted tumor proliferation and progression [39].
4.4. CSC Differentiation into Neurons
Cancer stem cells (CSCs) are a group of undifferentiated tumor cells that possess self-renewal, multipotency, and differentiation capabilities. CSCs play a critical role in driving tumorigenesis, metastasis, and recurrence [92,93]. One study demonstrates that the neurotransmitter 5-hydroxytryptamine (5-HT) produced by enteric serotonergic neurons is able to modulate the self-renewal and tumor-initiation capacities of colorectal CSCs [94]. Lu et al. observed that neural cells with human cell-specific markers were detectable in xenografts after the transplantation of CSCs from gastric and colorectal cancer patients into nude mice. In an in vitro differentiation assay, they provided experimental evidence that a fraction of CSCs has the capacity to generate neuronal cells. These CSC-derived neural cells express tyrosine hydroxylase (TH) and vesicular acetylcholine transporter (VaChT), the neuronal markers of sympathetic and parasympathetic neurons, respectively [95]. The notion that CSCs serve as a source of tumor neurogenesis was further supported via RNA sequencing analyses of aldehyde dehydrogenase (ALDH)-positive CSCs from colon cancer patient-derived organoids (PDOs) and xenografts (PDXs). The study confirmed an enrichment of neural developmental gene expression in CSCs. Moreover, the functional analyses demonstrated a key role for the neural crest stem cell (NCSC) regulator early growth response 2 (EGR2) in tumor growth, suggesting that targeting EGR2 may provide a therapeutic differentiation strategy to eliminate CSCs and block nervous system-driven disease progression [96].
4.5. Macrophage Transdifferentiation into Neurons
In addition to neuronal cells, cancer pain is also associated with numerous non-neuronal cells mainly through the secretion of pro-inflammatory mediators or algogenic mediators to sensitize nociceptors in the TME [99]. Monocytes and macrophages can produce tumor necrosis factor (TNF) and interleukin 1 beta (IL-1β) to enhance pain transduction and conduction, and the depletion of these cells conversely impairs the development of mechanical and thermal hypersensitivity [100,101]. Taken together, these results suggest that tumor-associated macrophages (TAMs), as an important component of the TME, have the potential to modulate intra-tumoral neural infiltration. Based on this hypothesis, Tang et al. used the single-cell RNA sequencing of lung adenocarcinoma to uncover a macrophage-to-neuron-like cell transition (MNT), describing a phenomenon in which TAMs directly transdifferentiate into neuron-like cells to facilitate de novo neurogenesis [102].
. Neuro–Immune Interactions
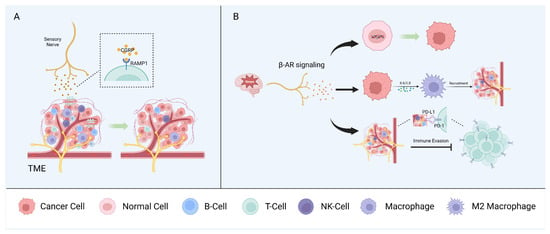
Mutual interactions between the nervous and immune systems play a critical role in maintaining homeostasis. In the context of cancer, neurons and immune and tumor cells interact via soluble signaling molecules and neurotransmitters to form a triangular relationship that modulates tumor growth and progression. However, sensory nerves, autonomic nerves, and other neural components have diverse effects on the regulation of the immune phenotype and the anti-tumor immune response [1,104] (Figure 3).

Figure 3. Neuron–immune interactions. Sensory nerves (A) and autonomic peripheral nerves (B) interact with immune components of the TME and promote tumor growth synergistically.
5.1. Sensory Nerves
Nociceptors are the most widely studied sensory nerve fibers in the context of the immune surveillance and immune escape of cancers. Nociceptors can influence immune components by releasing neuropeptides, such as CGRP, and, in melanoma, CGRP released by nociceptors directly promotes CD8+ T cell exhaustion [104,105]. Indeed, the pharmacological nociceptor inhibition or blocking of the receptor activity modifying protein 1 (RAMP1), the receptor for CGRP, reduced T cell exhaustion and inhibited tumor growth in a mouse model. CD8+ T cell exhaustion was restored in a mouse model in which most mechano- and thermosensitive nociceptors were ablated via treatment with recombinant CGRP. Analysis of single-cell RNA sequencing data from human melanoma samples showed that CD8+ T cells expressing RAMP1 are more likely to be exhausted than RAMP1-negative cells and are associated with a poor prognosis [106]. CGRP also suppresses the murine macrophage antigen presentation to T cells by modulating the cytokine expression and regulating macrophage polarization to the pro-tumorigenic M2 subtype [107].
In the head and neck region, tissues are innervated by sensory nerves, primarily originating from the trigeminal ganglia [39,108]. CGRP, as the most abundant neurotransmitter in trigeminal ganglion neurons [108], has been shown to regulate the immune response through the RAMP1 signaling pathway in OSCC [109]. This study demonstrated a higher expression of RAMP1 for tumor-infiltrating immune cells (CD4+ T cells, cytotoxic CD8+ T cells, and NK cells) in OSCC patients compared to immune cells from the normal tissue of healthy individuals [109]. High expression of RAMP1 was also confirmed in cultured oral cancer cells and orthotopic xenografts, contributing to oral cancer pain [110]. In Cgrp knock-out mice, tumor growth was significantly inhibited compared to wildtype controls, accompanied by an increase in tumor-infiltrating immune cells [109].
5.2. Autonomic Peripheral Nerves
Stress can promote tumor initiation and progression in several cancer types, including oral, prostate, and skin cancers [112,113]. This is explained, in part, by the fact that the stress-induced adrenergic signaling produced by local sympathetic nerves has multiple effects on different immune components of the TME. Accordingly, stress has been linked with increased metastasis in breast cancer, in part by affecting the recruitment of tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and other immune cells [114].
Stress-induced β-adrenergic receptor (β-AR) signaling causes DNA damage, leading to tumorigenic effects in human oral keratinocytes [115]. In ovarian cancer cells, exposure to norepinephrine (NE) causes DNA double-strand breaks, and pretreatment with propranolol (a non-selective β-blocker) can counteract the norepinephrine-induced DNA damage [116].
5.3. Other Neural Components
Schwann cells (SCs) are another notable member of the peripheral nervous system that can produce chemokines to enhance the chemotactic capacity of immune cells [124]. SCs modulate the immune microenvironment through the CCL2/ C-C motif chemokine receptor 2 (CCR2) axis. CCL2 released by SCs enhances the proliferation, migration, and invasion capabilities and EMTs of tumor cells, as well as induces TAMs to polarize into the M2 subtype, resulting in immunosuppression [125,126]. Toll-like receptors (TLRs), the activation of which modulates the maturation of antigen-presenting cells and T cell activation, are also expressed on SCs [104,127,128].
6. Imaging and Treatment Strategy
6.1. Imaging Technologies for Cancer–Neuron Interactions
To better understand the interactions between the PNS and tumor, multidisciplinary techniques and methods are required to provide clear insights from the morphological to the molecular level. At the structural and functional level, electron microscopy (EM) is widely used to reveal the synaptic structures between neurons and tumor cells [131]. Dynamic contrast-enhanced magnetic resonance (MR) and MR spectroscopy are utilized for the neuroimaging of in vivo models or clinical diagnosis [132,133]. In CNS tumors, multiphoton laser-scanning microscopy (MPLSM), which provides high-resolution in vivo imaging, has been used to monitor the coordinated activity of tumor cells in neuronal signaling when combined with calcium imaging in a spatial and functional manner [131,134,135].
6.2. Neuroscience-Instructed Therapeutic Approaches
The emerging clinical relevance of the reciprocal cancer–neuron interactions within the TMEs of many human tumors has attracted great interest in the development of innovative therapeutic strategies, either based on the identification of new drug targets or the repurposing of already well-established drugs for anti-tumor treatment. For example, β-blockers are widely used as drugs to regulate blood pressure, heart rate, and airway reactivity [138], but they also show anti-tumor effects due to their antagonistic actions on the adrenergic nervous system [39,139,140]. In breast cancer, a phase II clinical trial demonstrated that propranolol decreased the expressions of biomarkers, which are associated with EMT-related signaling and metastasis in treated patients [141].
Local denervation via surgical or pharmacological intervention is another potential strategy to treat cancer patients, especially those with neurotropic carcinomas. Microsurgical denervation, which minimizes the extent of surgical intervention, is considered an alternative for cancer patients with a high risk of undergoing direct tumor resection [14]. In prostate cancer, a phase I/II clinical trial performing denervation via unilateral botulinum toxin type A injection prior to prostatectomy showed increased tumor cell apoptosis compared to contralateral tumor tissue [142].
In head and neck cancer, the translation of PNS-targeted therapy from preclinical models to clinical practice is limited, in part because the abundant and complex nerve distribution in this anatomic region restricts the adequate inhibition of neurons. The development of new targeted therapies based on neuron-specific strategies requires a better understanding of the underlying principles of the complex cancer–neuron crosstalk.
This entry is adapted from the peer-reviewed paper 10.3390/cells13030256
This entry is offline, you can click here to edit this entry!
