Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Natural killer (NK) cells and cytotoxic T (CD8+) cells are two of the most important types of immune cells in our body, protecting it from deadly invaders. While the NK cell is part of the innate immune system, the CD8+ cell is one of the major components of adaptive immunity. Still, these two very different types of cells share the most important function of destroying pathogen-infected and tumorous cells by releasing cytotoxic granules that promote proteolytic cleavage of harmful cells, leading to apoptosis.
- NK subpopulation
- natural killer cell
- cytotoxic T cell
- microorganism
- granules
- innate immunity
- adaptive immunity
- immunosenescence
1. Natural Killer (NK) Cells and Their Subpopulations
Natural killer (NK) cells are a vital part of our immune system’s defense against pathogens and cancer. They originate from bone marrow, lymph nodes, and liver in the form of NK cell precursors, which differentiate into NK cells and no other lineages [1]. The cytokine IL-15 is essential for NK cell development and homeostasis. While NK cells are considered major components of the immune system in protecting the body against pathogens and cancer, like macrophages, neutrophils, and the like, they are neither considered B nor T cells and are best described as innate cytotoxic lymphocytes with adaptive immune features, so that they are now considered a bridge between innate and adaptive immunity [2][3][4][5][6][7]. They constitute about 5–15% of all lymphocytes circulating in the blood, which makes them the third largest lymphocyte population, after T and B cells. Humans with deficient or dysfunctional NK cells are susceptible to infections that can potentially be life-threatening [8]. NK cells contribute to host defense in several ways, both directly and indirectly [9][10].
What distinguishes NK cells from other immune cells is their inherent ability to respond rapidly to pathogens without prior activation. Unlike many immune cells that require a specific stimulus or activation signal to initiate a response, NK cells are primed to detect and attack invading pathogens immediately upon their entry into the body, without any priming or prior activation by other cells. Instead, NK cells basically have a form of friend-or-foe identification system. For the NK cell, the friend identifier is the major histocompatibility complex I (MHC-I) molecule on the suspect cell’s surface [11][12]. If the NK cell finds an MHC-I molecule on the surface of a suspect cell, it will not destroy that cell. If an NK cell does not find an intact MHC-I on a target cell, it will go into kill mode and destroy that cell. However, there is a third scenario wherein a target cell still has an MHC-I molecule but also expresses a ligand, such as a viral antigen, that identifies it as a harmful cell. In this case, the ligand overrides the inhibitory “friend” signal from the MHC-I, and the NK cell goes into kill mode on the target cell [11][12].
This ability to tell friend from foe is part of what makes NK cells extremely efficient at finding and killing viruses, cancers, and the cells infected by them. This is also what makes NK cells so good at their job as part of the body’s first line of defense against pathogens. NK cells also kill bacteria, fungi, and parasites that invade the human body, either killing these microbes directly or the cells that they have infected [13][14][15][16][17]. Low numbers of NK cells are associated with increased prevalence of fungal infection, and there is a correlation between reduced NK cell cytotoxic activity and fungal infections [18]. NK cells have been shown to be active and cytotoxic against bacteria such as Salmonella typhimurium [19], Shigella flexneri [20], Mycobacterium tuberculosis [21], Staphylococcus aureus [22], Listeria monocytogenes [23], and Pseudomonas aeruginosa [24], and against mold and fungi such as Candida albicans [25][26], Cryptococcus neoformans [26][27], Aspergillus fumigatus [28][29], Paracoccidioides braziliensis [30], Coccidioides immitis [31], Bacillus anthracis [32], and Pneumocystis murina [33].
Studying the various ways in which NK cells make use of the many different components of cellular toxicity may provide valuable information about defending against harmful microorganisms [34].
The immunophenotyping of the different NK and cytotoxic T-cell subpopulations is made possible because the surfaces of these cells express special molecules called cellular differentiation or cluster differentiation (CD) markers. Included in these markers are the receptors and ligands that enable cytotoxic protective immune cells to tell normal, harmless, or host cells from diseased, harmful, or pathogenic organisms [35]. These CD markers have been identified and numbered and are used to distinguish the different NK-cell and T-cell subpopulations from each other.
Among the different receptors found on their surfaces, NK cells carry three major CD markers: CD16, CD56, and CD57. Based on the amount, strength, and concentration of the markers found on their cell membrane, NK cells can be classified into three basic subsets [14][36].
1.1. What Are CD56+CD16dim NK Cells?
CD56brightCD16neg/dim or CD56+CD16− NK cells make up about 10% of NK cells and are considered immature. These NK cells are found mainly in the lymphoid tissues. Despite the fact that they are only weakly cytotoxic, they are called regulatory NK cells because they are abundant producers of cytokines and chemokines such as interferon-gamma (IFN-γ), tumor necrosis factor beta (TNF-β), interleukin-10 (IL-10), and IL-13, giving them immuno-regulatory properties and a role in shaping the adaptive immune response. This cytokine-producing ability has been found to be negatively affected in people with myeloma. Phenotypic alterations of the CD56+ immune cell fraction have been reported in patients with various infectious autoimmune or malignant diseases [14][15][16].
1.2. What Are CD56dimCD16+ NK Cells?
CD56dimCD16+ or CD56−CD16+ NK cells constitute about 90% of circulating NK cells and are considered mature. These are cytotoxic NK cells that are involved in the cytotoxicity of viral-infected cells and tumor cells. They are also involved in cytokine production. Most NK cells express the activating CD16 receptor. They also express Fc-gamma receptors that engage the Fc region of IgG [37]. The CD16 receptor lacks the ability to signal on its own; therefore, it requires an adaptor molecule that contains an immunoreceptor tyrosine-based activating motif (ITAM) to mediate signaling cascades. In NK cells, cross-linking of the CD16 receptor results in phosphorylation of the ITAM(s) that initiate signaling cascades, which ultimately lead to the release of cytotoxic granules containing granzymes and perforin, a process known as antibody-dependent cellular cytotoxicity (ADCC), leading to killing of target cells. When an antibody binds to a receptor on a tumor cell, the CD56−CD16+ NK cell binds to the antibody, allowing the NK cell to release its cytotoxic granules to kill the cell. Likewise, if an anti-EBV antibody binds to a receptor on an EBV-infected cell, the CD56−CD16+ NK cell can bind to that antibody, induce the release of granules, and kill the virus-infected cell [37].
1.3. What Are CD57+ Cells?
CD57 is a marker protein found on the surface of a subset of NK cells. They are a highly mature or developed, discrete, functionally distinct subpopulation of CD16+ NK cells that are involved in immunoprotecting against pathogens and other environmental factors. Also called memory NK cells, they display high cytotoxic potential and are highly differentiated long-lived cells that are thought to be more efficient at killing their targets compared to other NK-cell subpopulations. CD57+ NK cells have become a matter of recent interest because they are now believed to play a unique role in immune function and have been implicated in a number of health conditions [36][37][38][39][40][41][42][43][44]. The CD57 marker is also detected on neural cells, in which it acts as an adhesion molecule, and thus may be involved in the communications between the nervous system and the immune system [45]. CD57+ NK cells are consistently associated with better outcomes in cancer and autoimmune disease. Low levels of CD57+ are associated with lower overall survival in cancer [46][47][48][49][50][51][52][53][54][55][56][57][58][59].
CD57 is a very useful marker of NK-cell maturation. Studies show that progression from CD56bright to CD56dimCD57− to CD56dimCD57+ reflects a maturation pathway for NK cells [4]. Acquisition of CD57 means a higher cytotoxic capacity and greater responsiveness to signaling via CD16 and natural cytotoxic receptors. CD57+ cells identify the final stage of NK cell maturation, and their numbers increase with aging [36][39][60][61]. The numbers of CD57+ NK cells also greatly increase in response to chronic exposure to antigens originating from tumor cells, bacteria, and viruses, particularly with EBV, CMV, HIV, hepatitis C, and more. Increases in the detectable numbers of CD57+ NK cells in the blood are also associated with autoimmune disease [36][48][49].
In different autoimmune diseases, CD57+ NK cells fulfill an immunoregulatory role with their ability to delete autoreactive T cells that are chronically activated by viral antigens. Generally, increases in the population of autoreactive CD57+ cells are associated with more severe autoimmune diseases such as Wegener’s granulomatosis [62], multiple sclerosis (MS) [63], type 1 diabetes [64], Graves’ disease [65], and rheumatoid arthritis [66]. However, there are some instances where certain autoimmune diseases are consistently associated with reduced frequencies or absolute numbers of circulating CD57+ NK cells and/or impaired cytotoxicity [67][68][69][70][71][72][73][74][75]. In several autoimmune diseases such as atopic dermatitis [76][77], Sjögren’s syndrome [78], IgA nephropathy [79], psoriasis [80], and alopecia areata [81], a reduction in CD57+ NK cells in peripheral blood was reported in comparison to controls.
CD57+ NK cells are very important in early pregnancy failure. In fact, NK cell counts were shown to be greater in a subgroup of patients who suffered recurrent pregnancy failure (3.42% ± 2.15) compared to controls (2.14% ± 1.42) [39].
In relation to many pathogens that induce or cause an increase in CD57+ cells, in 2001, it was reported that there was a reduction in the blood levels of CD57+ NK cells in patients with chronic Lyme disease in comparison to those with acute Lyme disease and uninfected individuals [82][83]. However, a 2009 study found no differences between the NK cell counts of patients with chronic Lyme disease and those of controls [84]. In agreement with this article, many authorities such as the CDC [85], North American medical experts [86], European science organizations [87], and the Royal College of Pathologists of Australasia [88] have explicitly warned against the use of CD57 and other unvalidated tests in the diagnosis of Lyme disease. Thus, CD57+ cells should be measured not for the diagnosis of Lyme disease but for the indication of chronic exposure to antigens that originate from bacteria, viruses, tumor cells, and neoantigens formed in the body. The counting of CD57+ cells is also recommended for patients with various autoimmune diseases, as the majority of them are induced by environmental factors [36].
1.4. What Are CD57+CD16+ NK Cells?
CD57+CD16+ cells are a subpopulation of CD56dimCD16+ NK cells that carry CD57 markers of their surfaces. Cells that carry both CD57 and CD16 markers are highly cytotoxic.
CD57+CD16+ cells contain the cytolytic enzymes granzyme A, granzyme B, and perforin in their granules, enabling them to kill cancer cells and virus-infected cells with great efficiency. These cells are considered the most potent cells for combating acute and chronic viral infection [36][38].
-
Elevation in CD57+CD16+ cells is an indication of persistent antigenic stimulation, especially from viruses. Human CMV is one of the clearest examples of infection driving NK cell differentiation, particularly driving the expansion of NKG2C+ NK cells, which preferentially acquire CD57. Mature CD57+ NK cells expand as a consequence of lifetime exposure to infections, including HBV, HCV, EBV, CMV, hantavirus, and HIV. Their increase is an indication of ongoing or previous viral infection. Elevation in the levels of CD57+ NK cells observed in patients with chronic viral infection correlates with slower disease progression [36][38].
Compared to healthy controls, in patients with severe COVID-19, high frequencies of CD57+CD16+ were detected despite the absolute number of these cells being decreased due to low WBC and low lymphocyte counts. Individuals with long COVID showed significantly increased levels of functional memory cells with high antiviral cytotoxic activity, including NK cells with the CD56+CD57+NKG2C+ phenotype. The hypothesis that a persistent memory cytotoxic response against SARS-CoV-2 could be the cause of long COVID is supported by NK cells expressing both memory (CD57) and activation (NKG2C) markers that did not go away even after the initial SARS-CoV-2 infection was cleared [89][90][91][92][93].
In autoimmune diseases, reduced frequencies or absolute numbers of circulating CD57+ NK cells and/or impaired NK-cell cytotoxicity have consistently been observed. This supports the regulatory role of cytotoxic CD57+ NK cells in preventing or suppressing autoimmune disease. For example, the blood level of CD57+CD16+ cells is depleted in inflammatory and some autoimmune disorders [63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83], such as psoriasis [73], atopic dermatitis [76][77], rheumatoid arthritis [69], and multiple sclerosis [67][68]. However, an increase in the number of CD57+ CD16+ cells has been shown in tissues, such as the skin, joints, and pancreas, of affected individuals. Increased oxidative stress in patients with SLE may have caused preferential apoptosis of mature CD56dimCD57+ NK cells, perhaps contributing to the impairment of the ability to eliminate pathogenic CD4+ T cells seen in SLE [41][71][78][94]. This impairment in the number and cytotoxic capacity of CD57+CD16+ NK cells may be due to various mechanisms that different pathogens employ in the induction of some autoimmune diseases.
2. Cytotoxic CD8+ T Cells or Cytotoxic T Lymphocytes (CD8+, CTL, TC)
CD8+ T cells, like NK cells, originate in the bone marrow. However, what makes these cells different is that they migrate to the thymus and develop into maturity there, which is where the “T” in their name comes from [95][96]. They include cytotoxic T cells, which are the main cellular warriors of the adaptive immune system, specializing in killing virally infected or malignant cells. They are the adaptive counterparts of the innate NK cells [97]. They are called cytotoxic lymphocytes because they express cytotoxic molecules such as granzyme A, granzyme B, and perforin. They also produce IFN-γ and TNF-α. These functions and properties together enable them to kill viral-infected cells and cancer cells with a high degree of efficiency. They are considered the most potent cells for stopping the spread of pathogens and tumor cells [98].
In order for cytotoxic CD8+ T cells to kill their pathogenic target cells, they first have to be activated or primed by antigen-presenting cells, which give them little pieces of the pathogen’s material so that the CD8+ T cells can identify their targets. Cytotoxic CD8+ T cells are very efficient in the killing pathogens, particularly viruses that live in the cell. Cells harboring such viruses express on their surface viral peptides bound to major histocompatibility complex I (MHC-I) molecules that are detectable by the cytotoxic CD8+ T cell [47][97][98][99].
Thus, NK cells and CD8+ T cells are a complementary pairing, cytotoxic partners in defeating pathogenic cells. Since NK cells are from the innate immune system and CD8+ T cells are from the adaptive immune system, they are, as Rosenberg and Huang call them, parallel and complementary soldiers of immunotherapy [100].
2.1. Important CD8+ T-Cell Subsets
CD57+ CD8+ T cells are a critical subpopulation of CD8+ T cells that are mediators of adaptive immunity. CD8+ is formidable enough alone, but putting CD8+ together with CD57+ to obtain CD57+CD8+ is like combining two highly effective tools into one master tool. There is growing evidence that the CD57+CD8+ T-cell population plays a significant role in various diseases or conditions associated with chronic immune activation [47]. As a result of lifetime exposure to common antigens such as pathogens, neoantigens, and autoantigens, the numbers of CD57+CD8+ cells increase with age. This is because persistent exposure to these antigens induces the expansion of CD57+CD8+ cells [101][102][103].
An increase in the CD57+CD8+ T-cell population is observed in individuals with chronic infections such as EBV, CMV, measles, hepatitis C, parvovirus, HIV, SARS-CoV-2, toxoplasma, and more.
An increase in the numbers of CD57+CD8+ T cells has been observed in the blood of patients with different malignancies [40][41][42][43][44][45][46][47][48][49][50][51], including melanomas, head and neck cancer, and hemato-oncological diseases.
Quantitative changes in the CD57+CD8+ T-cell population are observed in different autoimmune diseases, such as MS [67][68], type 1 diabetes [64], Graves’ disease [65], ankylosing spondylitis [47], and rheumatoid arthritis [66][69]. In Graves’ disease [65], ankylosing spondylitis [47], polymyositis [47], dermatomyositis [47][74], and rheumatoid arthritis [66], increased CD57+CD8+ T cells are also associated with the severity of the disease. In rheumatoid arthritis, after treatment with abatacept, a decrease in CD57+CD8+ T cells correlated with clinical response [104]. In other autoimmune diseases, including lupus, type 1 diabetes [63], and MS [67][68], decreases in the number of CD57+CD8+ T cells were observed. It has also been observed that CD8+CD57+ T cells in tumors lack cytotoxic activity, although the boosting of IL-15 was able to restore the impaired proliferative activity of CD8+CD57+ T cells in tumors and peripheral blood [105].
And finally, increases in the numbers of CD57+CD8+ T cells have been observed in chronic pulmonary disease [106], chronic alcoholism [47], organ and bone marrow transplantation [107], and acute physical stress [47].
Another subset of CD8+ T cells is composed of KIR+CD8+ cells, which are present in lymph nodes, spleen, and peripheral blood and make up to 4.5% of total T cells in healthy adults [108]. These cells exert immunosuppressive and immunoregulatory capabilities. KIR+CD8+ cells exhibit high levels of cytotoxic molecules such as granzyme B and perforin [109]. The population of these cells expands during viral infection, cancer, aging, and many autoimmune disorders [109][110][111][112]. In relation to autoimmune diseases and infections, Li et al. [112] not only found increased frequencies of KIR+CD8+ in celiac disease and in patients with coronavirus infection but also demonstrated immunosuppression and immunoregulatory function against pathogenic CD4+ T cells. They concluded that development of autoimmunity caused by viral infection could be prevented by KIR+CD8+ regulatory T cells. Furthermore, in a very recent article, Paris-Muñoz et al. discussed the importance of the Helios IKZF2 gene transcription factor and its influence on KIR+CD8+ Tregs and other immunosuppressive cells in transfer therapies as a potential treatment for systemic lupus erythematosus (SLE) and other autoimmune disorders [113]. These immunosuppressive and immunoregulatory capabilities of KIR+CD8+ Tregs and their importance in preventing the viral initiation of autoimmunity through the induction of programmed cell death in autoreactive Th1 and Th17 pathogenic T cells are shown in Figure 1.
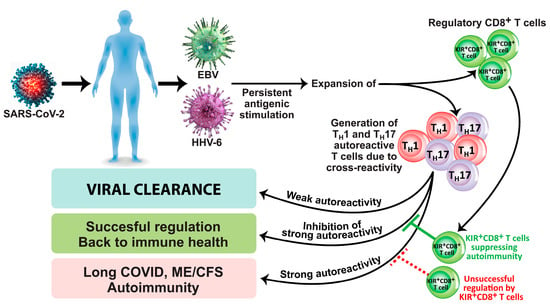
Figure 1. Induction of long COVID, ME/CFS, and autoimmunity due to viral infection/reactivation in the absence of successful regulation by KIR+CD8+ regulatory T cells.
2.2. What Are CD3+CD16+CD56+ NKT Cells?
CD3+CD16+CD56+ cytotoxic natural killer T cells or NKT cells constitute a unique and very rare subset of CD1d-restricted T cells that serve as a bridge between innate and adaptive immunity because they carry receptors characteristic of both T cells and NK cells. They have major modulating effects on immune responses via the secretion of cytokines. NKT cells are considered important players in tumor immunosurveillance, and the have been shown to play a role in immune protection against microbial pathogens, in different types of cancers, and in the control of autoimmune diseases [114][115].
Th1-like NKT cells can induce an antitumor response, while Th2- and Treg-like NKT-cell subsets facilitate immune escape and tumor progression. An important subset of NKT cells comprises the invariant NKT or iNKT cells. These CD1d-dependent NKT cells express an invariant T-cell receptor alpha chain. While NKT cells generally come to be exhausted in advanced cancer, iNKT cells actually increase in activation and effector function within the breast tumor microenvironment [116][117]. The number of circulatory NKT cells is significantly decreased in patients with different cancers compared to healthy controls. Low NKT-cell numbers correlated with poor clinical outcomes in patients with some kinds of cancer [118]. Metelitsa et al. analyzed 98 untreated primary neuroblastomas from patients with stage 4 metastatic disease and found that, in relationship to iNKT infiltration, survival at 5 years was 64% for iNKT+ and 35% for iNKT− tumors (p = 0.007) [119]. Peng et al. tested fresh peripheral blood from 63 never-treated patients with gastric cancer and normal blood from 30 healthy control individuals using flow cytometry [120]. They found that the frequencies of CD3+CD56+ NKT-like cells were significantly lower in tumors (4.44%) compared to those in non-tumor tissues (7.20%). Molling et al. tested heparinized blood samples from 69 healthy subjects and 120 advanced cancer patients, none of whom had received chemotherapy or radiotherapy at the time of analysis, and found that cancer patients showed a 47% reduction in circulating NKT cells (p = 0.013) [121]. In another study also headed by Molling [122], circulating iNKT cells were evaluated in 47 patients with head and neck squamous cell carcinoma or HNSCC prior to radiotherapy. Clinical data obtained after a follow-up period of 31 months divided the patients into three separate groups with a small, intermediate, or large circulating iNKT cell fraction. These three groups were significantly associated with a decreased 3-year overall survival rate (39%, 75%, and 92%, respectively) and with a disease-specific survival rate (43%, 87%, and 92%, respectively). Klatka et al. [123] performed immunophenotyping on peripheral blood samples from 40 men and 10 women with laryngeal cancer in stages ranging from stage I to stage IV. The healthy control group consisted of 12 men and 3 women. Patients with advanced laryngeal cancer showed a significantly lower percentage of iNKT cells than controls (0.08% vs. 0.23%, p = 0.00046). These and many other demonstrations of low NKT levels correlating with poor outcomes in different kinds of cancer were summarized by Krijgsman et al. [118].
Alterations in the numbers and functions of NKT cells have also been associated with SSc, T1D, MS, and other autoimmune diseases [118][124][125][126][127][128][129][130][131][132]. Too many NKT cells could be the result of a strong immune response against microbial pathogens as a protective mechanism during the early phase of infections. High NKT frequency could be due to abnormally high NKT development in the thymus, an increased rate of basal proliferation, or an increased rate of survival in the periphery. In some autoimmune diseases in which a phospholipid was the target antigen, an increase in the number of NKT cells was observed [132]. This is because cardiolipin is among the reported lipid antigens recognized by CD1d [114][132]. Furthermore, increased numbers of CD3−CD56+ NK and CD3+CD56+ NKT cells were observed in patients with COPD compared to controls [130]. These numbers are an indication not only of immune dysfunction in COPD but also of the participation of NK and NKT cells in the pathogenesis of COPD. Elevated levels of circulating CD3+CD16+CD56+ cells were found in pregnant women and were associated with an increased rate of pregnancy and live birth in in vitro fertilization treatments [133].
The clinical significance of these seven major immune cell subpopulations and how they protect the body is summarized in Table 1.
Table 1. Characteristics of human NK cells and CD8+ T cells and their clinical significance.
| Immunophenotype | Cytotoxic Activity | Clinical Significance | References |
|---|---|---|---|
| CD56bright(+)CD16− NK Cell | Weakly cytotoxic Produces limited amounts of cytotoxic molecules |
Immunoregulation through production of many cytokines and chemokines | [134][135][136][137][138][139][140][141] |
| CD56−CD16bright(+) NK Cell | Naturally strongly cytotoxic Produces significant amounts of cytotoxic molecules |
Protection against microorganisms and cancerous cells | [142][143][144][145][146][147][148][149][150] |
| CD3−CD57bright(+) NK Cell | High cytotoxic potential as a result of producing various cytotoxic molecules | Long-lived cells Immunoprotection against pathogens, especially during aging Deletion of autoreactive T cells Prevention of some autoimmune diseases |
[142][143][144][145][146][147][148][149][150][151][152] |
| CD57bright(+) CD16bright(+) NK Cell | Highly cytotoxic Produces significant amounts ofcytotoxic molecules |
The most potent cells for combating acute and chronic infections | [57][59][151][152][153][154][155][156][157] |
| CD8+ Cytotoxic T Cell | After activation by APC, becomes highly cytotoxic Produces different cytotoxic molecules |
Immune response to bacterial/viral infections and cancer |
[102][158][159][160][161][162][163][164][165][166][167] |
| CD8+CD57+ T Cell | Highly cytotoxic Produces significant amounts of cytotoxic molecules |
Mediators of adaptive immunity Associated with chronic immune activation Age-related changes in the immune system status |
[47][48][49][69][103][168][169][170] |
| CD3+CD56+CD16+ T Cell | Moderately cytotoxic Plays a significant role in immunoregulation |
Serves as bridge between innate and adaptive immunity Immune protection against microbial pathogens and cancer Control of autoimmune diseases |
[118][125][126][127][128][130][131] |
These important subsets of immune cells are shown in Figure 2.
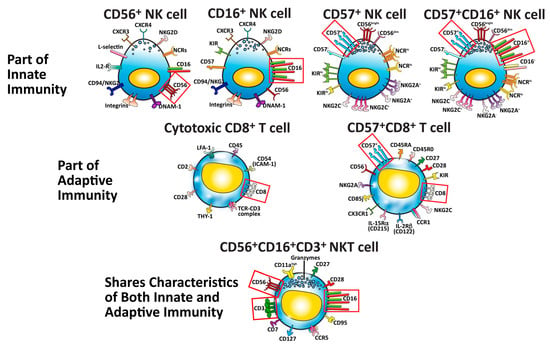
Figure 2. Seven major cells that protect our body against pathogens, cancers, and neo-antigens. The identifying cluster differentiation (CD) markers for each specific cell type are outlined in red boxes.
2.3. How the Innate and Adaptive Immune Systems Work Together to Protect the Body against Different Pathogens
To better understand the division of labor between NK and cytotoxic T cells in protecting the body against pathogens, the researchers have summarized this information in the following seven steps (see Figure 3):
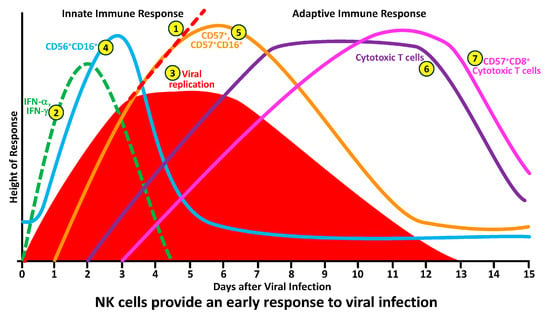
Figure 3. How NK and cytotoxic T-cell subpopulations respond during the early and late phases of infection.
-
In the absence of a functional innate and adaptive immune response, pathogens in infected cells or in their tissue environment grow exponentially. This exponential growth of the pathogens may result in serious inflammation, a cytokine storm, and death.
-
Upon the detection of pathogens by antigen-presenting T-helper, NK cells, cytotoxic T cells and NKT cells become activated and produce significant amounts of IFN-γ within minutes. The job of interferon is to stop the spread of the pathogens before the NK cells and cytotoxic T cells arrive at the battleground.
-
This production of IFN-γ by different cells inhibits pathogen replication, resulting in the flattening of the pathogen curve until the NK cells arrive at the site of the infection.
-
The arrival of CD56+ and CD16+ NK cells at the infected area not only stops the replication of pathogens but also causes a significant decline in their numbers by killing the pathogens and the pathogen-infected cells.
-
To finish the job, CD57+ and CD57+CD16+ cells, which are highly cytotoxic, are now summoned to join the operation in order to cleanse the body of the pathogen’s remnants, and, thus, the immune system prevails against infections.
-
All these steps of pathogen elimination are part of the innate immune response in which the responding cytotoxic activity is carried out within minutes to hours. However, if the NK cell and its subpopulations fail to destroy the pathogen entirely, the immune system must call on additional resources.
-
These are the complementary components of adaptive immunity, such as cytotoxic T cells, and their hybrids with CD57, the CD57+CD8+ cytotoxic T cells, will arrive at the pathogen-infected area within a few days of infection.
This is how the NK-cell and cytotoxic T-cell subpopulations respond during the early and late phases of infection by different microorganisms (see Figure 3).
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms12010230
References
- Bozzano, F.; Perrone, C.; Moretta, L.; De Maria, A. NK cell precursors in human bone marrow in health and inflammation. Front. Immunol. 2019, 10, 02045.
- Lau, C.M.; Adams, N.M.; Geary, C.D.; Weizman, O.E.; Rapp, M.; Pritykin, Y.; Leslie, C.S.; Sun, J.C. Epigenetic control of innate and adaptive immune memory. Nat. Immunol. 2018, 19, 963–972.
- Mujal, A.M.; Delconte, R.B.; Sun, J.C. Natural killer cells: From innate to adaptive features. Annu. Rev. Immunol. 2021, 39, 417–447.
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561.
- Santosa, E.K.; Kim, H.; Rückert, T.; Le Luduec, J.-B.; Abbasi, A.J.; Wingert, C.K.; Peters, L.; Frost, J.N.; Hsu, K.C.; Romagnani, C.; et al. Control of nutrient uptake by IRF4 orchestrate innate immune memory. Nat. Immunol. 2023, 24, 1685–1697.
- Basilio-Queiros, D.; Mischak-Weissinger, E. Natural killer cells—From innate cells to the discovery of adaptability. Front. Immunol. 2023, 14, 1172437.
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49.
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–526.
- Schmidt, S.; Tramsen, L.; Rais, B.; Ulrich, E.; Lehrnbecher, T. Natural killer cells as a therapeutic tool for infectious diseases—Current status and future perspectives. Oncotarget 2018, 9, 20891–20897.
- Schmidt, S.; Ulrich, E.; Bochennek, K.; Zimmermann, S.-Y.; Lehrnbrecher, T. Role of natural killer cells in antibacterial immunity. Expert Rev. Hematol. 2016, 9, 1119–1127.
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124.
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502.
- Mody, C.H.; Ogbomo, H.; Xiang, R.F.; Kyei, S.K.; Feehan, D.; Islam, A.; Li, S.S. Microbial killing by NK cells. J. Leukoc. Biol. 2019, 105, 1285–1296.
- Lodoen, M.B.; Lanier, L.L. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006, 18, 391–398.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510.
- Garcia-Penarrubia, P.; Koster, F.T.; Kelley, R.O.; McDowell, T.D.; Bankhurst, A.D. Antibacterial activity of human natural killer cells. J. Exp. Med. 1989, 169, 99–113.
- Ogbomo, H.; Mody, C.H. Granule-dependent natural killer cell cytotoxicity to fungal pathogens. Front. Immunol. 2016, 7, 692.
- Stuehler, C.; Kuenzli, E.; Jaeger, V.K.; Baettig, V.; Ferracin, F.; Rajacic, Z.; Kaiser, D.; Bernardini, C.; Forrer, P.; Weiiser, M.; et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation and association with occurrence and outcome of invasive aspergillosis. J. Infect. Dis. 2015, 212, 959–967.
- Tagliabue, A.; Nencioni, L.; Villa, L.; Boraschi, D. Genetic control of an in vitro- natural cell-mediated activity against Salmonella typhimurium by intestinal and splenic lymphoid cells in mice. Clin. Exp. Immunol. 1984, 56, 531–536.
- Klimpel, G.R.; Niesel, D.W.; Klimpel, K.D. Natural cytotoxic effector cell activity against Shigella flexneri-induced HeLa cells. J. Immunol. 1986, 136, 1081–1086.
- Lu, C.C.; Wu, T.S.; Hsu, Y.J.; Chang, C.J.; Lin, C.S.; Chia, J.H.; Wu, T.L.; Huang, T.T.; Martgel, J.; Ojcius, D.M.; et al. NK cells kill mycobacteria directly by releasing perforin and granulysin. J. Leukoc. Biol. 2014, 96, 1119–1129.
- Small, C.L.; McCormick, S.; Gill, N.; Kugathasan, K.; Santosuosso, M.; Donaldson, N.; Heinrichs, D.E.; Ashkar, A.; Xing, Z. NK cells play a critical protective role in host defense against acute extracellular Staphyulococcus aureus bacterial infection in the lung. J. Immunol. 2008, 180, 5558–5568.
- Naper, C.; Shegarfi, H.; Inngjerdingen, M.; Rolstad, B. The role of natural killer cells in the defense against Listeria monocytogenes lessons from a rat model. J. Innate Immun. 2011, 3, 289–297.
- Feenan, D.D.; Jamil, K.; Polyal, M.J.; Ogbomo, H.; Hasell, M.; Li, S.S.; Xiang, R.F.; Parkins, M.; Trapani, J.A.; Harrison, J.J.; et al. Natural killer cells kill extracellular Pseudomonas aeruginosa using contact-dependent release of granzymes B and H. PLoS Pathog. 2022, 18, e1010325.
- Voigt, J.; Kurzai, O. The role of natural killer cells in the immune response against Candida albicans. Mycoses 2011, 54, 413–414.
- Li, S.S.; Ogbomo, H.; Mansour, M.K.; Xiang, R.F.; Szabo, L.; Munro, F.; Mukherjee, P.; Mariuzza, R.A.; Amrein, M.; Vyas, J.M.; et al. Identification of the fungal ligand-triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat. Commun. 2018, 9, 751.
- Ma, L.L.; Wang, C.L.; Neely, G.G.; Epelman, S.; Krensky, A.M.; Mody, C.H. NK cells use perforin rather than granulysin for anticryptococcal activity. J. Immunol. 2004, 173, 3357–3365.
- Schmidt, S.; Tramsen, L.; Hanisch, M.; Latgé, J.P.; Huenecke, S.; Koehl, U.; Lehrnbecher, T. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J. Infect. Dis. 2011, 203, 430–435.
- Bouzani, M.; Ok, M.; McCormick, A.; Ebel, F.; Kurzai, O.; Morton, C.O.; Einsele, H.; Loeffler, J. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-γ release. J. Immunol. 2011, 187, 1369–1376.
- Jimenez, B.E.; Murphy, J.W. In vitro effects of natural killer cells against Paracoccidioides brasiliensis yeast phase. Infect. Immun. 1984, 46, 552–558.
- Petkus, A.F.; Baum, L.L. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J. Immunol. 1987, 139, 3107–3111.
- Gonzales, C.M.; Williams, C.B.; Calderon, V.E.; Huante, M.B.; Moen, S.T.; Popov, V.L.; Baze, W.B.; Peterson, J.W.; Endsley, J.J. Antibacterial role for natural killer cells in host defense to Bacillus anthracis. Infect. Immun. 2012, 80, 234–242.
- Kelly, M.N.; Zheng, M.; Ruan, S.; Kolls, J.; D’Souza, A.; Shellito, J.E. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol. 2013, 190, 285–295.
- Pierce, S.; Geanes, E.S.; Bradley, T. Targeting natural killer cells for improved immunity and control of the adaptive immune response. Front. Cell. Infect. Microbiol. 2020, 10, 231.
- Xiong, S.; Xu, W. Cluster of differentiation. In Comprehensive Biomedical Physics; Brahme, A., Ed.; Elsevier: Stockholm, Sweden, 2014; Volume 10, pp. 91–114.
- Nielsen, C.M.; White, M.J.; Goodier, M.R.; Riley, E.M. Functional significance of CD57 expression on human NK cells and relevance to disease. Front. Immunol. 2013, 4, 422.
- Aguilar, O.A.; Gonzalez-Hinojosa, M.D.R.; Arakawa-Hoyt, J.S.; Millan, A.J.; Gotthardt, D.; Nabekura, T.; Lanier, L.L. The CD16 and CD32b Fc-gamma receptors regulate antibody-mediated responses in mouse natural killer cells. J. Leukoc. Biol. 2023, 113, 27–40.
- Lopez-Vergés, S.; Milush, J.M.; Pandey, S.; York, V.A.; Arakawa-Hoyt, J.; Pircher, H.; Norris, P.J.; Nixon, D.F.; Lanier, L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010, 116, 3865–3874.
- Björkström, N.K.; Strunz, B.; Ljungren, H.-G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2022, 22, 112–123.
- Ozcimen, E.E.; Kiyici, H.; Uckuyu, A.; Yanik, F.F. Are CD57+ natural killer cells really important in early pregnancy failure? Arch. Gynecol. Obstet. 2009, 279, 493–497.
- Lu, Z.; Tian, Y.; Bai, Z.; Liu, J.; Zhang, Y.; Qi, J.; Jin, M.; Zhu, J.; Li, X. Increased oxidative stress contributes to impaired peripheral CD56dimCD57+ NK cells from patients with systemic lupus erythematosus. Arthritis Res. Ther. 2022, 24, 48.
- Song, K.; Coleman, R.A.; Alber, C.; Ballas, Z.K.; Waldschmidt, T.J.; Mortari, F.; LaBrecque, D.R.; Cook, R.T. TH1 cytokine response of CD57+ T-cell subsets in healthy controls and patients with alcoholic liver disease. Alcohol 2001, 24, 155–167.
- Youn, J.C.; Jung, M.K.; Yu, H.T.; Kwon, J.S.; Kwak, J.E.; Park, S.H.; Kim, I.C.; Park, M.S.; Lee, S.K.; Choi, S.W.; et al. Increased frequency of CD4+CD57+ senescent T cells in patients with newly diagnosed acute heart failure: Exploring new pathogenic mechanisms with clinical relevance. Sci. Rep. 2019, 9, 12887.
- Kiu, B.; Yang, G.X.; Sun, Y.; Tomiyama, T.; Zhang, W.; Leung, P.S.C.; He, X.S.; Dhaliwal, S.; Invernizzi, P.; Gershwin, M.E.; et al. Decreased CD57 expression of natural killer cells enhanced cytotoxicity in patients with primary sclerosing cholangitis. Front. Immunol. 2022, 13, 912961.
- Künemund, V.; Jungalwala, F.B.; Fischer, G.; Chou, D.K.; Keilhauer, G.; Schachner, M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J. Cell Biol. 1988, 106, 123–213.
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271.
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+CD28− and CD8+CD57+ T cells and their role in health and disease. Immunology 2011, 134, 17–32.
- Characiejus, D.; Pasukoniene, V.; Kazlauskaite, N.; Valuckas, K.P.; Petraitis, T.; Mauricas, M.; Den Otter, W. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res. 2002, 22, 3679–3683.
- Characiejus, D.; Pasukoniene, V.; Jonusauskaite, R.; Azlauskaite, N.; Aleknavicius, E.; Mauricas, M.; Den Otter, W. Peripheral blood CD8highCD57+ lymphocyte levels may predict outcome in melanoma patients treated with adjuvant interferon-alpha. Anticancer Res. 2008, 28, 1139–1142.
- Akagi, J.; Baba, H. Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer. Int. J. Clin. Oncol. 2008, 13, 528–535.
- Sze, D.M.; Brown, R.D.; Yuen, E.; Gibson, J.; Ho, J.; Raitakari, M.; Basten, A.; Joshua, D.E.; de St Groth, B.F. Clonal cytotoxic T cells in myeloma. Leuk. Lymphoma 2003, 44, 1667–1674.
- Van den Hove, L.E.; Vandenberghe, P.; Van Gool, S.W.; Ceuppens, J.L.; Demuynck, H.; Verhoef, G.E.; Boogaerts, M.A. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: Evidence for systemic activation of the T cell compartment. Leuk. Res. 1998, 22, 175–184.
- Atayar, C.; Poppema, S.; Visser, L.; van den Berg, A. Cytokine gene expression profile distinguishes CD4+/CD57+ T cells of the nodular lymphocyte predominance type of Hodgkin’s lymphoma from their tonsillar counterparts. J. Pathol. 2006, 208, 423–430.
- Serrano, D.; Monteiro, J.; Allen, S.L.; Kolitz, J.; Schulman, P.; Lichtman, S.M.; Buchbinder, A.; Vinciguerra, V.P.; Chiorazzi, N.; Gregersen, P.K. Clonal expansion within the CD4+CD57+ and CD8+CD57+ T cell subsets in chronic lymphocytic leukemia. J. Immunol. 1997, 158, 1482–1489.
- Di Girolamo, W.; Coronato, S.; Portiansky, E.; Laguens, G. Profile of immune cells in lymph nodes draining human malignant tumors. Medicina 2008, 68, 423–427.
- Muntasell, A.; Servitjia, S.; Cabo, M.; Bermejo, B.; Pérez-Buira, S.; Rojo, F.; Costa-Garcia, M.; Arpi, O.; Moraru, M.; Serrano, L.; et al. High numbers of circulating CD57+ NK cells associate with resistance to HER2- specific therapeutic antibodies in HER2+ primary breast cancer. Cancer Immunol. Res. 2019, 7, 1280–1292.
- Taghavi, N.; Bagheri, S.; Akbarzadeh, A. Prognostic implication of CD57, CD16 and TGF-β expression in oral squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 58–62.
- Stabile, H.; Fionda, C.; Gismondi, A.; Santoni, A. Role of distinct natural killer cell subsets in anticancer response. Front. Immunol. 2017, 8, 293.
- Chattopadhyay, P.K.; Betts, M.R.; Price, D.A.; Gostick, E.; Horton, H.; Roederer, M.; De Rosa, S.C. The cytolytic enzymes granyzme A, granzyme B, and perforin: Expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 2009, 85, 88–97.
- Hazeldine, J.; Lord, J.M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev. 2013, 12, 1069–1078.
- Rodriguez, I.J.; Ruiz, N.L.; Leon, M.L.; Enriquez, L.M.; Velasquez, M.P.M.; Aguirre, J.P.O.; Bohorquez, O.M.R.; Vargas, E.A.V.; Hernandez, E.D.; Lopez, C.A.P. Immunosenescence study of T cells: A systematic review. Front. Immunol. 2021, 11, 604591.
- Giscombe, R.; Wang, X.B.; Kakoulidou, M.; Lefvert, A.K. Characterization of the expanded T-cell populations in patients with Wegener’s granulomatosis. J. Intern. Med. 2006, 260, 224–230.
- Ratts, R.B.; Karandikar, N.J.; Hussain, R.Z.; Choy, J.; Northrop, S.C.; Lovett-Racke, A.E.; Racke, M.K. Phenotypic characterization of autoreactive T cells in multiple sclerosis. J. Neuroimmunol. 2006, 178, 100–110.
- Mikulkova, Z.; Praksova, P.; Stourac, P.; Bednarik, J.; Strajtova, L.; Pacasova, R.; Belobradkova, J.; Dite, P.; Michalek, J. Numerical defects in CD8+CD28- T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis. Cell. Immunol. 2010, 262, 75–79.
- Sun, Z.; Zhong, W.; Lu, X.; Shi, B.; Zhu, Y.; Chen, L.; Zhang, G.; Zhang, X. Association of Graves’ disease and prevalence of circulating IFN-gamma-producing CD28(-) T cells. J. Clin. Immunol. 2008, 28, 464–472.
- Wang, E.C.; Lawson, T.M.; Vedhara, K.; Moss, P.A.; Lehner, P.J.; Borysiewicz, L.K. CD8high+(CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1997, 40, 237–248.
- Takahashi, K.; Miyake, S.; Kondo, T.; Terao, K.; Hatakenaka, M.; Hashimoto, S.; Yamamura, T. Natural killer type2 bias in remission of multiple sclerosis. J. Clin. Investig. 2001, 107, R23–R29.
- Kastrukoff, L.F.; Morgan, N.G.; Zecchini, D.; White, R.; Petkau, A.J.; Satoh, J.; Paty, D.W. A role for natural killer cells in the immunopathogenesis of multiple sclerosis. J. Neuroimmunol. 1998, 86, 123–133.
- Aramaki, T.; Ida, H.; Izumi, Y.; Fujikawa, K.; Huang, M.; Arima, K.; Tamai, M.; Kamachi, M.; Nakamura, H.; Kawakami, A.; et al. A significantly impaired natural killer cell activity due to a low activity on a per-cell basis in rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 245–252.
- Izumi, Y.; Ida, H.; Huang, M.; Iwanaga, N.; Tanaka, F.; Aratake, K.; Arima, K.; Tamai, M.; Kamachi, M.; Nakamura, H.; et al. Characterization of peripheral natural killer cells in primary Sjogren’s syndrome: Impaired NK cell activity and low NK cell number. J. Lab. Clin. Med. 2006, 147, 242–249.
- Park, Y.W.; Kee, S.J.; Cho, Y.N.; Lee, E.H.; Lee, H.Y.; Kim, E.M.; Shin, M.H.; Park, J.J.; Kim, T.J.; Lee, S.S.; et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 1753–1763.
- Ciampolillo, A.; Guastamacchia, E.; Amati, L.; Magrone, T.; Munno, I.; Jirillo, E.; Triggiani, V.; Fallacara, R.; Tafaro, E. Modifications of the immune responsiveness in patients with autoimmune thyroiditis: Evidence for a systemic immune alteration. Curr. Pharm. Des. 2003, 9, 1946–1950.
- Cameron, A.L.; Kirby, B.; Griffiths, C.E. Circulating natural killer cells in psoriasis. Br. J. Dermatol. 2003, 149, 160–164.
- O’Gorman, M.; Smith, R.; Garrison, A.; Shamiyeh, E.; Pachman, L. Lymphocyte subsets in peripheral blood from newly diagnosed, untreated patients with juvenile dermatomyositis (JDM) are associated with disease activity scores (DAS). Arthritis Rheum. 2002, 46, S490.
- Wouters, C.H.P.; Ceuppens, J.L.; Stevens, E.A.M. Different circulating lymphocyte profiles in patients with different subtypes of juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 2002, 20, 239–248.
- Wehrmann, W.; Reinhold, U.; Kukel, S.; Franke, N.; Uerlich, M.; Kreysel, H.W. Selective alterations in natural killer cell subsets in patients with atopic dermatitis. Int. Arch. Allergy Appl. Immunol. 1990, 92, 318–322.
- Matsumura, G. Leu7(HNK-1)-positive cells in peripheral blood and natural killer cell activity in patients with atopic dermatitis. Nihon Hifuka Gakkai Zasshi 1990, 100, 57–62.
- Struyf, N.J.; Snoeck, H.W.; Bridts, C.H.; De Clerck, L.S.; Stevens, W.J. Natural killer cell activity in Sjogren’s syndrome and systemic lupus erythematosus: Stimulation with interferons and interleukin-2 and correlation with immune complexes. Ann. Rheum. Dis. 1990, 49, 690–693.
- Antonaci, S.; Polignano, A.; Ottolenghi, A.; Tortorella, C.; Schena, F.P. Redistribution of natural killer (NK) cell frequency and NK cytotoxic activity in primary IgA nephropathy. Cytobios 1992, 69, 27–34.
- Batista, M.D.; Ho, E.L.; Kuebler, P.J.; Milush, J.M.; Lanier, L.L.; Kallas, E.G.; York, V.A.; Chang, D.; Liao, W.; Unemori, P.; et al. Skewed distribution of natural killer cells in psoriasis skin lesions. Exp. Dermatol. 2013, 22, 64–66.
- Imai, R.; Miura, J.; Numata, K.; Aikawa, Y.; Takamori, K.; Ogawa, H. Analysis of T cell, activated T cell and NK cell subsets in peripheral blood lymphocytes from patients with alopecia areata. In Trends in Human Hair Growth and Alopecia Research; Van Neste, D., Lachapelle, J.M., Antoine, J.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 299–304.
- Stricker, R.B.; Winger, E.E. Decreased CD57 lymphocyte subset in patients with chronic Lyme disease. Immunol. Lett. 2001, 76, 43–48.
- Stricker, R.B.; Burrascano, J.; Winger, E. Longterm decrease in the CD57 lymphocyte subset in a patient with chronic Lyme disease. Ann. Agric. Environ. Med. 2002, 9, 111–113.
- Marques, A.; Brown, M.R.; Fleisher, T.A. Natural killer cell counts are not different between patients with post-Lyme disease syndrome and controls. Clin. Vaccine Immunol. 2009, 16, 1249–1250.
- Laboratory Tests and Practices That Are Not Currently Recommended. Available online: https://www.cdc.gov/lyme/diagnosistesting/labtest/otherlab/index.html (accessed on 27 December 2023).
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaeerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme disease. Clin. Infect. Dis. 2021, 72, e1–e48.
- Mygland, A.; Ljostad, U.; Fingerle, V.; Rupprecht, T.; Schmutzhard, E.; Steiner, I. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur. J. Neurol. 2010, 17, 8–16.
- The Royal College of Pathologists of Australasia. Position Statement: Diagnostic Laboratory Testing for Lyme Disease (or Similar Syndromes) in Australia and New Zealand; Microbiol AC, 1/2014; 2022. Available online: https://www.rcpa.edu.au/Library/College-Policies/Position-Statements/Diagnostic-Laboratory-testing-for-Borreliosis-Lyme (accessed on 27 December 2023).
- Galán, M.; Vigón, L.; Fuentes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent overactive cytotoxic immune response in a Spanish cohort of individuals with long COVID: Identification of diagnostic biomarkers. Front. Immunol. 2022, 13, 848886.
- Di Vito, C.; Calcaterra, F.; Colaniz, N.; Terzoli, S.; Voza, A.; Mikulak, J.; Della Bella, S.; Mavilio, D. Natural killer cells in SARS-CoV-2 infection: Pathophysiology and therapeutic implications. Front. Immunol. 2022, 13, 888248.
- Clavarino, G.; Leroy, C.; Epaulard, O.; Raskovalova, T.; Vilotitch, A.; Pernollet, M.; Dumestre-Pérard, C.; Defendi, F.; Le Maréchal, M.; Le Gouellec, A.; et al. Fine analysis of lymphocyte subpopulations in SARS-CoV-2 infected patients: Differential profiling of patients with severe outcome. Front. Immunol. 2022, 13, 889613.
- Varchetta, S.; Mele, D.; Oliviero, B.; Mantovani, S.; Ludovisi, S.; Cerino, A.; Bruno, R.; Castelli, A.; Mosconi, M.; Vecchia, M.; et al. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 604–612.
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020, 5, eabd6832.
- Li, H.; Geng, L.; Cao, Z.; Jin, Z.; Yan, Q.; Long, X.; Zhang, X.; Meng, D.; Jiang, Y.; Li, W.; et al. CD56brightCD16− to CD57+CD56dimCD16+ NK cell ratio discriminates disease activity and renal involvement in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2023, 41, 1768–1776.
- Masopust, D.; Vezys, V.; Wherry, E.J.; Ahmed, R. A brief history of CD8 T cells. Eur. J. Immunol. 2007, 37, S103–S110.
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001.
- Cassioli, C.; Baldari, C.T. The expanding arsenal of cytotoxic T cells. Front. Immunol. 2022, 13, 883010.
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367.
- Jaime-Sanchez, P.; Uranga-Murillo, I.; Aguilo, N.; Khouili, S.C.; Arias, M.A.; Sancho, D.; Pardo, J. Cell death induced by cytotoxic CD8+ T cells is immunogenic and primes caspase-3-dependent spread immunity against endogenous tumor antigens. J. Immunother. Cancer 2020, 8, e000528.
- Rosenberg, J.; Huang, J. CD8+ T cells and NK cells: Parallel and complementary soldiers of immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20.
- Chijioke, O.; Landtwing, V.; Münz, C. NK cell influence on the outcome of primary Epstein-Barr virus infection. Front. Immunol. 2016, 7, 323.
- van den Berg, S.P.H.; Pardiek, I.N.; Lanfermeijer, J.; Sauce, D.; Klenerman, P.; van Baarle, D.; Arens, R. The hallmarks of CMV-specific CD8 T-cell differentiation. Med. Microbiol. Immunol. 2019, 208, 365–373.
- Bandres, E.; Merino, J.; Vasquez, B.; Inoges, S.; Moreno, C.; Subira, M.L.; Sanchez-Ibarrola, A. The increase of IFN-γ production through aging correlates with the expanded CD8+highCD28-CD57+ subpopulation. Clin. Immunol. 2000, 96, 230–235.
- Sato, M.; Takemura, M.; Shinohe, R.; Yamamoto, Y.; Saito, K. A study of natural killer cell subtractions in Abatacept therapy for rheumatoid arthritis. In Proceedings of the ACR/ARHP Annual Meeting, San Diego, CA, USA, 25–30 October 2013. Abstract 1385.
- Huang, B.; Liu, R.; Wang, P.; Yuan, Z.; Yang, J.; Xiong, H.; Zhang, N.; Huang, Q.; Fu, X.; Sun, W.; et al. CD8+CD57+ T cells exhibit distinct features in human non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e000639.
- Olloquequi, J.; Montes, J.F.; Prats, A.; Rodríguez, E.; Montero, M.A.; García-Valero, J.; Ferrer, J. Significant increase of CD57+ cells in pulmonary lymphoid follicles of COPD patients. Eur. Resp. J. 2011, 37, 289–298.
- Mendes, A.V.; Kallas, E.G.; Benard, G.; Pannuti, C.S.; Menezes, R.; Dulley, F.L.; Evans, T.G.; Salomao, R.; Machado, C.M. Impact of cytomegalovirus and grafts versus host disease on the dynamics of CD57+CD28−CD8+ T cells after bone marrow transplant. Clinics 2008, 63, 667–676.
- Björkström, N.K.; Beziat, V.; Cichocki, F.; Liu, L.L.; Levine, J.; Larsson, S.; Koup, R.A.; Anderson, S.K.; Ljunggren, H.G.; Malmberg, K.J. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood 2012, 120, 3455–3465.
- Koh, J.Y.; Shin, E.C. KIRs mark killers suppressing autoimmunity. Immunity 2022, 55, 735–737.
- Chan, W.K.; Rujkijyanont, P.; Neale, G.; Yang, J.; Bari, R.; Das Gupta, N.; Holladay, M.; Rooney, B.; Leung, W. Multiplex and genome-wide analyses reveal distinctive properties of KIR+ and CD56+ T cells in human blood. J. Immunol. 2013, 191, 1625–1636.
- Campilo, J.A.; Martinez-Escribano, J.A.; Moya-Quiles, M.R.; Marin, L.A.; Muro, M.; Guerra, N.; Parrado, A.; Campos, M.; Frias, J.F.; Minguela, A.; et al. Increased expression of the natural killer cell inhibitory receptor CD94/NKG2A and CD158b on circulating and lesional T cells in patients with chronic plaque psoriasis. Br. J. Dermatol. 2006, 155, 318–324.
- Li, J.; Zaslavsky, M.; Su, Y.; Guo, J.; Sikora, M.I.; van Unen, V.; Christophersen, A.; Chiou, S.H.; Chen, I.; Li, J.; et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Sci. Immunol. 2022, 376, eabi9591.
- Paris-Muñoz, A.; León-Triana, O.; Pérez-Martinez, A.; Barber, D.F. Helios as a potential biomarker in systemic lupus erythematosus and new therapies based on immunosuppressive cells. Int. J. Mol. Sci. 2024, 25, 452.
- Hill, T.M.; Bezbradica, J.S.; Van Kaer, L.; Joyce, S. CD1d-Restricted Natural Killer T Cells; John Wiley & Sons, Ltd.: Chichester, UK, 2016.
- Almeida, J.S.; Casanova, J.M.; Santos-Rosa, M.; Tarazona, R.; Solana, R.; Rodrigues-Santos, P. Natural killer T-like cells: Immunobiology and role in disease. Int. J. Mol. Sci. 2023, 24, 2743.
- Liu, X.; Li, L.; Si, F.; Huang, L.; Zhao, Y.; Zhang, C.; Hoft, D.F.; Peng, G. NK and NKT cells have distinct properties and functions in cancer. Oncogene 2021, 40, 4521–4537.
- Jerud, E.S.; Bricard, G.; Porcxelli, S.A. CD1d-restricted natural killer T cells: Roles in tumor immunosurveillance and tolerance. Transfus. Med. Hemother. 2006, 33, 18–36.
- Krijgsman, D.; Hokland, M.; Kuppen, P.J.K. The role of natural killer T cells in cancer—A phenotypical and functional approach. Front. Immunol. 2018, 9, 367.
- Metelitsa, L.S.; Wu, H.W.; Wang, H.; Yang, Y.; Warsi, Z.; Asgharzadeh, S.; Groshen, S.; Wilson, S.B.; Seeger, R.C. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J. Exp. Med. 2004, 199, 1213–1221.
- Peng, L.S.; Mao, F.Y.; Zhao, Y.L.; Wang, T.T.; Chen, N.; Zhang, J.Y.; Cheng, P.; Li, W.-H.; Lu, Y.-P.; Teng, Y.-S.; et al. Altered phenotypic and functional characteristics of CD3+CD56+ NKT-like cells in human gastric cancer. Oncotarget 2016, 7, 55222–55230.
- Molling, J.W.; Kolgen, W.; Langendijk, J.A.; van der Vliet, H.J.; Boomsma, M.F.; Kruizenga, H.; Smorenberg, C.H.; Molenkamp, B.G.; Langendeijk, J.A.; Leemans, C.R.; et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int. J. Cancer 2005, 116, 87–93.
- Molling, J.W.; Langius, J.A.; Langendijk, J.A.; Leemans, C.R.; Bontkes, H.J.; van der Vliet, H.J.; von Blomberg, M.E.; Scheper, R.J.; van den Eertwegh, A.J.M. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J. Clin. Oncol. 2007, 25, 862–868.
- Klatka, J.; Grywalska, E.; Wasiak, M.; Markowicz, J.; Trojanowski, P.; Olszanski, W.; Rolinski, J. The percentage of iNKT cells among other immune cells at various clinical stages of laryngeal cancer. Postepy Hig. Med. Dosw. 2016, 70, 392–399.
- Guven, H.; Gilljam, M.; Chambers, B.J.; Ljunggren, H.G.; Christensson, B.; Kimby, E.; Dilber, M.S. Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): A potential source for cellular immunotherapy. Leukemia 2003, 17, 1973–1980.
- Kumar, A.; Suryadevara, N.; Hill, T.M.; Bezbradeica, J.S.; Van Kaer, L.; Joyce, S. Natural killer T cells: An ecological evolutionary developmental biology perspective. Front. Immunol. 2017, 8, 1858.
- Wu, L.; Van Kaer, L. Natural killer T cells in health and disease. Front. Biosci. 2011, 3, 236–251.
- Wu, L.; Van Kaer, L. Natural killer T cells and autoimmune disease. Curr. Mol. Med. 2009, 9, 4–14.
- Chan, A.C.; Serwecinska, L.; Cochrane, A.; Harrison, L.C.; Godfrey, D.I.; Berzins, S.P. Immune characterization of an individual with an exceptionally high natural killer T cell frequency and her immediate family. Clin. Exp. Immunol. 2009, 156, 238–245.
- Tang, Y.; Li, X.; Wang, M.; Zou, Q.; Zhao, S.; Sun, B.; Xu, L.; Jiang, Y. Increased numbers of NK cells, NKT-like cells, and NK inhibitory receptors in peripheral blood of patients with chronic obstructive pulmonary disease. Clin. Dev. Immunol. 2013, 2013, 721782.
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: The peripheral blood immune cell profile. Cancer Immunol. Immunother. 2019, 68, 1011–1024.
- Sokhatska, O.; Padrão, E.; Sousa-Pinto, B.; Beltrão, M.; Mota, P.C.; Melo, N.; Delgado, L.; Morais, A. NK and NKT cells in the diagnosis of diffuse lung diseases presenting with a lymphocytic alveolitis. BMC Pulm. Med. 2019, 19, 39.
- Marrero, I.; Ware, R.; Kumar, V. Type II NKT cells in inflammation, autoimmunity, microbial immunity, and cancer. Front. Immunol. 2015, 6, 316.
- Zhou, J.; Zhao, X.; Wang, Z.; Wang, J.; Sun, H.; Hu, Y. High circulating CD3+CD56+CD16+ natural killer-like T cell levels predict a better IVF treatment outcome. J. Reprod. Immunol. 2013, 97, 197–203.
- Poli, A.; Michel, T.; Théresine, M.; Andrés, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465.
- Azuz-Lieberman, A.; Markel, G.; Mizrahi, S.; Gazit, R.; Hannah, J.; Achdout, H.; Gruda, R.; Katz, G.; Arnon, T.I.; Battat, S.; et al. The involvement of NK cells in ankylosing spondylitis. Int. Immunol. 2005, 17, 837–845.
- Marcenaro, E.; Notarangelo, L.D.; OIrange, J.S.; Vivier, E. Editorial: NK cell subsets in health and disease: New developments. Front. Immunol. 2017, 8, 1363.
- Nitta, T.; Yagita, H.; Sato, K.; Okumura, K. Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer-target cell interaction. J. Exp. Med. 1989, 170, 1757–1761.
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001, 97, 3146–3151.
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640.
- Almehmadi, M.; Flanagan, B.F.; Khan, N.; Alomar, S.; Christmas, S.E. Increased numbers and functional activity of CD56+ T cells in healthy cytomegalovirus positive subjects. Immunology 2014, 142, 258–268.
- Khakoo, S.I.; Thio, C.L.; Martin, M.P.; Brooks, C.R.; Gao, X.; Astemborski, J.; Cheng, J.; Goedert, J.J.; Vlahov, D.; Hilgartner, M.; et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 2004, 305, 872–874.
- Schafer, J.L.; Li, H.; Evans, T.I.; Estes, J.D.; Reeves, R.K. Accumulation of cytotoxic CD16+ NK cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J. Virol. 2015, 89, 6887–6894.
- Horowitz, A.; Strauss-Albee, D.M.; Leipold, M.; Kubo, J.; Nemat-Gorgani, M.; Dogan, O.C.; Dekker, C.L.; Mackey, S.; Maecker, H.; Swan, G.E.; et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 2013, 5, 208ra145.
- Müller-Durovic, B.; Grählert, J.; Devine, O.P.; Akbar, A.N.; Hess, C. CD56-negative NK cells with impaired effector function expand in CMV and EBV co-infected healthy donors with age. Aging 2019, 11, 724–740.
- Mavillo, D.; Lombardo, G.; Benjamin, J.; Kim, D.; Follman, D.; Marcenaro, E.; O’Shea, M.A.; Kinter, A.; Kovacs, C.; Moretta, A.; et al. Characterization of CD56-/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 2005, 102, 2886–2891.
- Gonzalez, V.D.; Falconer, K.; Björkström, N.K.; Blom, K.G.; Weiland, O.; Ljunggren, H.-G.; Alaeus, A.; Sandberg, J.K. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: Correlation with outcome of pegylated IFN-a and Ribavirin treatment. J. Immunol. 2009, 183, 6612–6618.
- Grier, J.T.; Forbes, L.R.; Monaco-Shawver, L.; Oshinsky, J.; Atkinson, T.P.; Moody, C.; Pandey, R.; Campbell, K.S.; Orange, J.S. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J. Clin. Investig. 2012, 122, 3769–3780.
- Hong, H.S.; Eberhard, J.M.; Keudel, P.; Bollmann, B.A.; Ballmaier, M.; Bhatnagar, N.; Zielinska-Skowronek, M.; Schmidt, R.E.; Meyer-Olson, D. HIV infection is associated with a preferential decline in less-differentiated CD56dim CD16+ NK cells. J. Virol. 2010, 84, 1183–1188.
- Hong, H.S.; Ahmad, F.; Eberhard, J.M.; Bhatnagar, N.; Bollmann, B.A.; Keudel, P.; Ballmaier, M.; Zielinska-Skowronek, M.; Schmidt, R.E.; Meyer-Olson, D. Loss of CCR7 expression on CD56bright NK cells is associated with a CD56dimCD16+ NK cell-like phenotype and correlates with HIV viral load. PLoS ONE 2012, 7, e44820.
- Björkström, N.K.; Ljunggren, H.G.; Sandberg, J.K. CD56 negative NK cells: Origin, function, and role in chronic viral disease. Trend Immunol. 2010, 31, 401–406.
- Björkström, N.K.; Riese, P.; Heuts, F.; Andersson, S.; Fauriat, C.; Ivarsson, M.A.; Björklund, A.T.; Flodström-Tullberg, M.; Michaëlsson, J.; Rottenberg, M.E.; et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010, 116, 3853–3864.
- Phetsouphanh, C.; Aldridge, D.; Marchi, E.; Munier, C.M.L.; Meyerowitz, J.; Murray, L.; Van Vuuren, C.; Goedhals, D.; Fidler, S.; Kelleher, A.; et al. Maintenance of functional CD57+ cytolytic CD4+ T cells in HIV+ elite controllers. Front. Immunol. 2019, 10, 1844.
- Lopez-Vergès, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.-M.; Norris, P.J.; et al. Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732.
- Gratama, J.W.; Kluin-Nelemans, H.C.; Langelaar, R.A.; den Ottolander, G.J.; Stijnen, T.; D’Amaro, J.; Torensma, R.; Tanke, H.J. Flow cytometric and morphologic studies of HNK1+ (Leu 7+) lymphocytes in relation to cytomegalovirus carrier status. Clin. Exp. Immunol. 1988, 74, 190–195.
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Vergès, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012, 119, 2665–2674.
- Foley, B.; Cooley, S.; Verneris, M.R.; Curtsinger, J.; Luo, X.; Waller, E.K.; Anasetti, C.; Weisdorf, D.; Miller, J.S. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J. Immunol. 2012, 189, 5082–5088.
- Wu, Z.; Sinzger, C.; Frascaroli, G.; Reichel, J.; Bayer, C.; Wang, L.; Schirmbeck, R.; Mertens, T. Human cytomegalovirus-induced NKG2Chi CD57hi natural killer cells are effectors dependent on humoral antiviral immunity. J. Virol. 2013, 87, 7717–7725.
- Jergovic, M.; Smithey, M.J.; Nikolich-Zugich, J. Intrinsic and extrinsic contributors to defective CD8+ T cell responses with aging. Exp. Gerontol. 2018, 105, 140–145.
- Mogilenko, D.A.; Shypnov, O.; Andhey, P.S.; Arthur, L.; Swain, A.; Esaulova, E.; Brioschi, S.; Shchukina, I.; Kerndl, M.; Bambouskova, M. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity 2021, 54, 99–115.e12.
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020, 21, 684–694.
- Ferreira, D.P.; Silva, J.G.; Wyss, T.; Marraco, S.A.F.; Scarpellino, L.; Charmoy, M.; Maas, R.; Siddiqui, I.; Tang, L.; Joyce, J.A.; et al. Central memory CD8+ T cells derive from stem-like Tcf7hi effector cells in the absence of cytotoxic differentiation. Immunity 2020, 53, 985–1000.
- Younes, S.-A.; Freeman, M.L.; Mudd, J.C.; Shive, C.L.; Reynaldi, A.; Panigrahi, S.; Estes, J.D.; Deleage, C.; Lucero, C.; Anderson, J.; et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Investig. 2016, 126, 2745–2756.
- Hassouneh, F.; Goldeck, D.; Pera, A.; van Heemst, D.; Slagboom, P.E.; Pawelec, G.; Solana, R. Functional changes of T-cell subsets with age and CMV infection. Int. J. Mol. Sci. 2021, 22, 9973.
- Almanzar, G.; Schmalzing, M.; Trippen, R.; Höfner, K.; Weißbrich, B.; Geissinger, E.; Meyer, T.; Liese, J.; Tomy, H.-P.; Prelog, M. Significant IFNγ responses of CD8+ T cells in CMV-seropositive individuals with autoimmune arthritis. J. Clin. Virol. 2016, 77, 77–84.
- Scotet, E.; Peyrat, M.-A.; Saulquin, X.; Retiere, C.; Couedel, C.; Davodeau, F.; Dulphy, N.; Toubert, A.; Bignon, J.-D.; Lim, A.; et al. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: Towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 1999, 29, 973–998.
- Schirmer, M.; Goldberger, C.; Würzner, R.; Duftner, C.; Pfeiffer, K.-P.; Clausen, J.; Neumayr, G.; Falkenbach, A. Circulating cytotoxic CD8+ CD28− T cells in ankylosing spondylitis. Arthritis Res. 2002, 4, 71–76.
- Tsukishiro, T.; Donnenberg, A.D.; Whiteside, T.L. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 2003, 52, 559–607.
- Morris, S.R.; Chen, B.; Mudd, J.C.; Panigrahi, S.; Shive, C.L.; Sieg, S.F.; Cameron, C.M.; Zidar, D.A.; Funderburg, N.T.; Younes, S.-A.; et al. Inflammescent CX3CR1+CD57+CD8+ T cells are generated and expanded by IL-15. JCI Insight 2020, 5, e132963.
- Cencioni, M.T.; Magliozzi, R.; Nicholas, R.; Ali, R.; Malik, O.; Reynolds, R.; Borsellino, G.; Battistini, L.; Muraro, P.A. Programmed death 1 is highly expressed on CD8+CD57+ T cells in patients with stable multiple sclerosis and inhibits their cytotoxic response to Epstein-Barr virus. Immunology 2017, 152, 660–676.
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; TGarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–443.
This entry is offline, you can click here to edit this entry!
